We recently found that gastric lesions generated by ischemia/reperfusion (I/R) were prevented by drugs that inhibit TXA2 production or blockade TXA2 TP receptors, suggesting the pathogenic importance of this prostanoid in this model (8, 9). Because TXA2 is known to potentiate the bradykinin-induced nociceptive response (10) and may be a mediator of epigastric pain (11), TXA2 antagonistic action is considered crucial to antiulcer drugs. We also set up a model of gastric bleeding induced by double antiplatelet therapy with low-dose aspirin (ASA) plus clopidogrel, a P2Y12 receptor antagonist (12), and found mucosal protective drugs to be effective in preventing the bleeding (13, 14). Since recent studies have reported a risk of adverse gastric reactions in patients taking antiplatelet drugs with nonsteroidal anti-inflammatory drugs (NSAIDs) (15, 16), it is important to find drugs effective in this model. Furthermore, clinical studies using capsule endoscopes or double-balloon endoscopes have confirmed that NSAIDs damage the small intestine at a higher incidence than previously thought (17-19). Since no satisfactory means for the prevention and treatment of these lesions are currently available, except for the use of PG analogs (19), the identification of effective therapies for the treatment of NSAID-induced small intestinal damage remains an urgent priority. Thus, it is critical to examine whether egualen has beneficial influences on these lesions induced in the stomach and the small intestine, to provide further information for clinical use of this drug in the treatment of gastrointestinal diseases.
In the present study, we examined the effects of egualen against gastric lesions produced by I/R as well as double antitplatelet therapy with ASA plus clopidogrel, and small intestinal lesions generated by loxoprofen in rats and investigated the possible mechanisms involved in the protective action of this drug.
Animals
Male C57BL/6 mice (3 months old; SLC, Shizuoka, Japan) and Sprague-Dawley rats (200–260 g; Nippon Charles River, Shizuoka, Japan) were acclimated to standard laboratory conditions (12:12 h light-dark cycle, temperature 22±1°C). Experiments were carried out with or without fasting, using four to six animals in a conscious state, unless otherwise specified. All experimental procedures involving animals were approved by the Experimental Animal Research Committee of the Kyoto Pharmaceutical University.
Induction of ischemia and reperfusion-induced gastric lesions
Acute gastric mucosal lesions were produced by I/R in 18 h-fasted mice, according to a recently published method (8). Briefly, under urethane anesthesia (1.25 g/kg i.p.), the celiac artery was clamped with a small clamp (disposable vascular clip, holding force 40 g; BEAR Medical Corporation, Chiba, Japan), and 30 min later reperfusion was achieved through removal of the clamp. Then, the animals were killed 60 min after the onset of reperfusion following ischemia for 30 min, and the stomach was excised, inflated by injecting 0.4 ml of 2% formalin for 10 min to fix the tissue walls, and opened along the greater curvature. Egalen (30 and 60 mg/kg), ozagrel (TXA2 synthase inhibitor: 200 mg/kg), or seratrodast (TXA2 antagonist: 3–30 mg/kg) was administered p.o. 60 min before ischemia. The area (mm2) of hemorrhagic lesions developed in the stomach was measured under a dissecting microscope (Olympus, Tokyo, Japan) with a square grid (x10). The person measuring the lesions did not know the treatments given to the animals. In some cases, the gastric mucosa was examined with a light microscope following I/R with or without egualen (60 mg/kg). The animals were killed 60 min after the onset of reperfusion following ischemia for 30 min, and the stomach excised. The tissue samples were then immersed in 10% neutralized formalin, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin (H&E).
Determination of myeloperoxidase (MPO) activity
MPO activity in the gastric mucosa was measured after I/R treatment in mice, according to a modified version of the method of Krawisz et al. (20). At 60 min after I/R-treatment, the animals were sacrificed by the withdrawal of blood from the heart through perfusion with saline, and the stomach was excised. After rinsing of the tissue with cold saline, the mucosa was scraped with glass slides, weighed, and homogenized in a 50 mM phosphate buffer containing 0.5% hexadecyl-trimethyl-ammonium bromide (HTAB; pH 6.0; Sigma). The homogenized samples were subjected to freezing and thawing three times, and centrifuged at 2,000 g for 10 min at 4°C. MPO activity in the supernatant was determined by adding 50 µl of the supernatant to 50 µl of 10 mM phosphate buffer (pH 6.0) and 50 µl of 1.5 M o-dianisidine hydrochloride (Sigma) containing 0.0005% (w/v) hydrogen peroxide. The changes in absorbance at 450 nm were recorded on a microplate reader (VERSAmax; Molecular Device, OA). Sample protein content was estimated by spectrophotometric assay (Pierce protein assay kit, IL), and the MPO activity was obtained from the slope of the reaction curve, based on the following equation: Specific activity (µmol H2O2/min/mg protein) = (OD/min)/OD/µmol H2O2 x mg protein). Egalen (30 and 60 mg/kg), ozagrel (TXA2 synthase inhibitor: 200 mg/kg) or seratrodast (TXA2 antagonist: 3–30 mg/kg) was administered p.o. 60 min before ischemia.
Gastric bleeding and lesions caused by ASA plus clopidogrel
An experimental system for effectively evaluating gastric ulcerogenic and bleeding responses to antiplatelet therapy was set up in 18 h-fasted rats under urethane anesthesia, according to a previously published paper (13). Briefly, two catheters were inserted into the rat stomach, one from an incision in the esophagus and another through the pylorus via an incision in the duodenum, and the stomach was then perfused with saline at a rate of 0.4 ml/min using an infusion pump, and the perfusate was collected every 5 min for determination of hemoglobin concentrations. After an equilibration period with saline perfusion for 2 hours, the stomach was perfused with 25 mM ASA plus 50 mM HCl for another 60 min. Clopidogrel (30 mg/kg) was given p.o. 24 hours before the perfusion. The doses of ASA and clopidogrel were determined based on our previous studies (13, 14). Gastric bleeding was evaluated as the hemoglobin concentration in the perfusate. At the end of the experiment (60 min after the onset of ASA+HCl perfusion) the animals were killed for examination of the gastric mucosa. The stomach was excised, treated with 2% formalin for fixation of the tissue walls, and then opened along the greater curvature, and the mucosa was examined for damage under a dissecting microscope (x10). The area (mm2) of macroscopically visible lesions was measured, summed for each tissue, and used as a lesion score. The person measuring the lesions did not know the treatments given to the animals. Egualen (10 and 30 mg/kg) or PGE2 (1 mg/kg) was given i.d. 60 min or i.v. 10 min before the ASA perfusion, respectively.
Determination of hemoglobin concentrations
Gastric bleeding was determined by an increase in the hemoglobin concentration of the gastric perfusate (13, 14). To this end the gastric perfusate was collected every 15 min during the experiment where the stomach was perfused with saline or 25 mM ASA in the presence of clopidogrel pretreatment (30 mg/kg). The concentration of hemoglobin was assessed on a Hitachi spectrophotometer (U-2000, Ibaraki, Japan), according to standard curves made by adding various amounts of rat hemoglobin (Sigma Chemicals, St. Louis, MO) to saline or 25 mM ASA solutions. When rat hemoglobin was dissolved in saline of pH 3.5, maximal absorption occurred at 386 nm (21). This wavelength was used for the estimation of hemoglobin in the gastric perfusates. Data were analyzed using the Short Softmax Program and the results were presented as micrograms per milliliter or micrograms per 90 min.
Induction of small intestinal damage by loxoprofen
Rats without fasting were administered loxoprofen (60 mg/kg) p.o. and killed 24 hours later under deep ether anesthesia (22). The small intestine was excised, treated with 2% formalin for 10 min to fix the tissue walls, and opened along the anti-mesenteric attachment. The area of macroscopically visible damage (mm2) was measured under a dissecting microscope with square grids (x10), summed per tissue, and used as a lesion score. To highlight hemorrhagic lesions, a 1% Evans blue solution was administered i.v. in a volume of 1 ml/animal 30 min before killing. The person measuring the lesions did not know the treatment given to the animals. Egualen was given p.o. twice daily (3–30 mg/kg: 9:30 AM and 8:30 PM) for 3 days before the administration of loxoprofen or acutely twice (10–100 mg/kg) 30 min before and 6 hours after loxoprofen. In some cases, the small intestine was examined with a light microscope following the administration of loxoprofen (60 mg/kg) with or without egualen (100 mg/kg). The animals were killed 24 hours after the loxoprofen treatment, and the small intestine was excised. The tissue samples were then immersed in 10% neutralized formalin, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin (H&E).
Determination of mucus secretion
The amount of mucus secreted in the small intestine was determined by periodic acid-Schiff (PAS) staining. Three hours after the administration of loxoprofen (60 mg/kg, p.o.), the animals were killed under deep diethyl ether anesthesia, and the small intestines were removed. The removed tissues were fixed in Carnoy’s fluid (ethanol: acetic acid: chloroform = 6:1:3) for 24 hours, embedded in paraffin, and sectioned at a thickness of 8 µm. PAS staining was performed according to the conventional method. Egualen (100 mg/kg) was given p.o. with or without co-administration of loxoprofen. In the combined administration, this agent was given p.o. 30 min before the administration of loxoprofen.
Determination of enterobacterial counts
Enterobacteria were enumerated according to a method described by Deitch et al. (23). Six hours after loxoprofen treatment (60 mg/kg, p.o.), the animals were killed under deep ether anesthesia, and the small intestines were removed. After each intestine was rinsed with sterile saline, the mucosa was scraped with glass slides, weighed, and homogenized in 1 ml of sterile phosphate-buffered saline (PBS) per 100 mg of wet tissue. Aliquots of the homogenate were placed on blood agar and Gifuco anaerobic medium agar (Nissui, Tokyo, Japan). Blood agar plates were incubated at 37°C for 24 hours under aerobic conditions (BBL Gas Pack Pouch Anaerobic System; BD Biosciences, San Jose, CA). Plates containing 10 to 300 colony-forming units (CFU) were examined for numbers of enterobacteria, and the data were expressed as log CFU per gram of tissue. Egualen (100 mg/kg) was given p.o. 30 min before the administration of loxoprofen.
Determination of iNOS mRNA expression
The expression of iNOS mRNA in the small intestinal mucosa was measured by reverse transcription-polymerase chain reaction (RT-PCR) (24). The animals were killed under deep ether anesthesia 6 hours after the administration of loxoprofen (60 mg/kg) p.o., and the small intestines were removed and stored at –80°C prior to use. Egualen (100 mg/kg) was given p.o. 30 min before the administration of loxoprofen. Total RNA was extracted from tissue samples using Sepasol RNA I (Nacalai Tesque, Kyoto, Japan). The total RNA was reverse-transcribed with a first strand cDNA synthesis kit (ReverTra Ace alpha, TOYOBO, Osaka, Japan). The sequences of the sense and antisense primers for rat iNOS and GAPDH and each product size are shown in Table 1. An aliquot of the RT reaction product served as a template with 30 s of denaturation at 95°C and 1 min of extension at 68°C using the Advantage 2 polymerase mixture (CLONTECH, Mountain View, CA) in a thermal cycler (PC-806, ASTEC, Fukuoka, Japan). A portion of the PCR mixture was electrophoresed on a 1.5% agarose gel in Tris-acetic acid-EDTA buffer (40 mM Tris, 20 mM acetic acid, and 2 mM EDTA; pH 8.1), and the gel was stained with ethidium bromide and photographed (BioDoc-It Imaging System, UVP, Upland, CA).
| Table 1. Sequences of sense and antisense primers for rat iNOS and GAPDH |
 |
Preparation of drugs
The drugs used were urethane (Tokyo Kasei, Tokyo, Japan), egualen Na (Ajinomoto Pharm Co., Kawasaki, Japan), ozagrel, prostaglandin E2 (Cayman Chemical, Ann Arbor, MI), seratrodast (LKT Laboratories, St. Paul, MN), clopidogrel (Sanofi-Aventis, Tokyo, Japan), and loxoprofen (Sigma Chemicals, St. Louis, MO). Loxoprofen was suspended in a hydroxypropylcellulose (HPC) solution (Wako Pure Chemicals, Osaka, Japan). Other agents were dissolved in saline. Each agent was prepared immediately before use and administered p.o. in a volume of 0.1 ml/20 g body weight or 1 ml/200 g body weight, respectively, in mice or rats, and i.d. or i.v. in a volume of 1 ml/200 g body weight or 0.1 ml/100 g body weight, respectively, in rats. Control animals received the vehicle in the same volume and via the same route.
Statistical analysis
Data are presented as the mean±S.E. for 5–7 mice or 4–13 rats per group. Statistical analyses were performed using a one-way analysis of variance (ANOVA) and Student’s t-test or Dunnett’s multiple comparison test where appropriate, and values of P<0.05 were considered significant.
Effect of egualen on ischemia/reperfusion-induced gastric damage in mice
Laparotomy without clamping of the gastric artery (sham operation) did not produce any damage in the mouse gastric mucosa. In the animals subjected to I/R treatment (30 min of ischemia followed by reperfusion for 60 min), however, multiple hemorrhagic lesions were observed in the gastric mucosa, the lesion score being 9.6.2±1.9~10.3±1.1 mm2 (Fig. 2). Pretreatment of the animals with ozagrel (200 mg/kg, p.o.), a TXA2 synthase inhibitor, significantly prevented the I/R-induced development of gastric lesions, the inhibition being 57.1%. Likewise, the severity of these lesions was dose-dependently reduced by prior administration of seratrodust (3–30 mg/kg), a TXA2 antagonist, and the effect was significant at 10 mg/kg or greater. Egualen (30 and 60 mg/kg) also dose-dependently and significantly mitigated the severity of the I/R-induced gastric damage, and the effect at 60 mg/kg was as potent as that of seratrodast at 30 mg/kg, the inhibition being 64.1%. In sham-operated animals without I/R treatment, no damage was detected even by histological observation (Fig. 3A). By contrast, severe damage was observed histologically in the stomach after I/R treatment; most of the damage was restricted to the surface epithelium, but some damage occurred deep in the mucosa (Fig. 3B). When the animals were pretreated with egualen (60 mg/kg, p.o.), the severity of the histological damage was markedly reduced, and only slight damage was observed in the surface epithelium (Fig. 3C).
 |
Fig. 1. Chemical structure of egualen Na (sodium 3-ethyl-7-isopropyl- 1-azulemesulfonate 1/3 hydrate). |
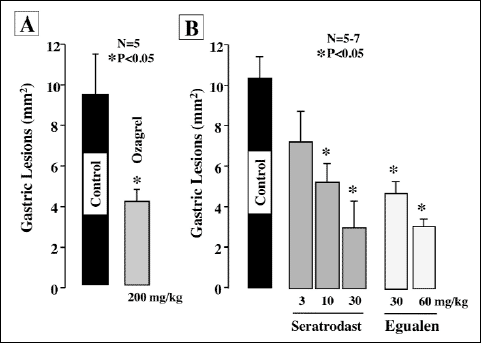 |
Fig. 2. Effects of egualen, ozagrel, and seratrodast on gastric lesions induced by I/R in mice. Under urethane anesthesia, the celiac artery was clamped. Reperfusion followed 30 min later with removal of the clamp, and then, the stomach was excised 60 min later. Egualen (3 and 60 mg/kg), ozagrel (200 mg/kg), or seratrodast (3–30 mg/kg) was given p.o. 30 min before the onset of ischemia. Data are presented as the mean±S.E. for 5~7 mice. *Significant difference from control, at P<0.05. |
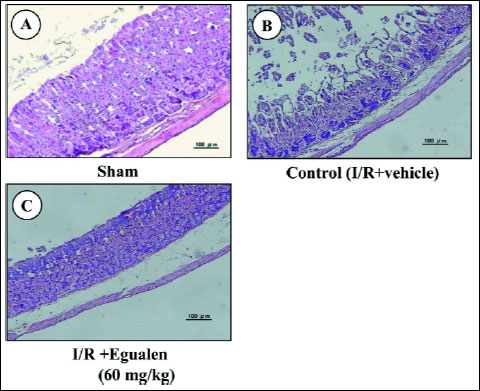 |
Fig. 3. Histological observations of I/R-induced gastric damage. Under urethane anesthesia, the celiac artery was clamped for 30 min. Reperfusion followed with removal of the clamp, and the stomach was excised after 60 min of reperfusion. Egualen (60 mg/kg) was given p.o. 30 min before the onset of ischemia. Fig. 3A shows: sham: Fig. 3B: control (I/R+vehicle); Fig. 3C: I/R plus egualen. |
Myeloperoxidase (MPO) activity
Gastric mucosal MPO activity in sham-operated mice was less than 0.02 µmol H2O2/min/mg protein. The MPO activity was markedly increased after I/R, the values being 0.044±0.007 µmol H2O2/min/mg protein. This response was significantly abrogated by the prior administration of egualen (60 mg/kg, p.o.), the inhibition being 51.5%. Both ozagrel (200 mg/kg, p.o.) and seratorodast (30 mg/kg) also suppressed the increase of MPO activity induced by I/R, the inhibition being 66.7% and 66.9%, respectively.
Effect of egualen on gastric bleeding and ulcerogenic responses to acidified ASA with or without clopidogrel pretreatment
Perfusion of the rat stomach with acidified ASA (25 mM in 50 mM HCl) produced few lesions, but the ulcerogenic response to acidified ASA was aggravated by pretreatment with clopidogrel (30 mg/kg). These treatments caused a time-dependent increase in the hemoglobin concentration, resulting in many hemorrhagic lesions in the stomach (Figs. 4 and 5A). PGE2 (1 mg/kg, i.v.) markedly reduced gastric bleeding and hemorrhagic damage induced by acidified ASA in the presence of clopidogel; the total hemoglobin output and hemorrhagic lesions were both reduced to about 20% of that in the vehicle-treated animals. Egualen (10 and 30 mg/kg, i.d.) dose-dependently reduced gastric bleeding and ulcerogenic responses to acidified ASA in the presence of clopidogel, and the effects were significant at 30 mg/kg. Notably, prior administration of egualen at 30 mg/kg also decreased the severity of lesions under such conditions almost as effectively as PGE2 at 1 mg/kg, the inhibition being 62.1%. As shown in Fig. 5B and Fig. 5C, the severity of gastric lesions produced by 25 mM ASA plus clopidogrel was markedly reduced by pretreatment with egualen (30 mg/kg), converting the lesions from hemorrhagic to non-hemorrhagic.
 |
| Fig. 4. Effects of egualen and PGE2 on gastric bleeding induced by luminal perfusion of acidified ASA in the presence of clopidogrel in urethane-anesthetized rats. The stomach was perfused by 25 mM ASA plus 50 mM HCl for 60 min. Clopidogrel (30 mg/kg) was given p.o. 24 hours before ASA. Egualen (30 and 60 mg/kg) or PGE2 (1 mg/kg) was given i.d. 60 min or i.v. 10 min, respectively, before the onset of ASA perfusion. Fig. A shows the time-course of change in the hemoglobin concentration in the luminal perfusate, and the data are presented as the mean±S.E. of values determined every 5 min from 6–8 rats. Fig. B shows total hemoglobin output in the perfusate for the last 60 min, and the data are presented as the mean±S.E. for 6–8 rats. Significant difference at P<0.05; * from control (without clopidogrel); # from vehicle. |
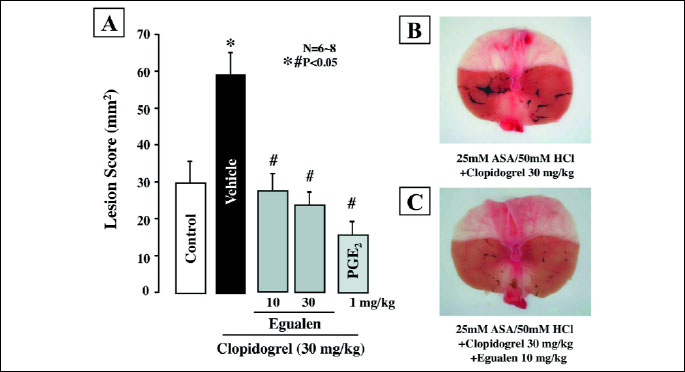 |
| Fig. 5. Effects of egualen and PGE2 on gastric hemorrhagic lesions produced by luminal perfusion of acidified ASA in the presence of clopidogrel in urethane-anesthetized rats. The stomach was perfused by 25 mM ASA plus 50 mM HCl for 60 min. Clopidogrel (30 mg/kg) was given p.o. 24 hours before ASA. Egualen (30 and 60 mg/kg) or PGE2 (1 mg/kg) was given i.d. 60 min or i.v. 10 min, respectively, before the onset of ASA perfusion. Fig. A shows hemorrhagic lesions, and the data are presented as the mean±S.E. for 6–8 rats. Significant difference at P<0.05; * from control (without clopidogrel); # from vehicle. Right panel shows macroscopic appearances of hemorrhagic gastric lesions induced by luminal perfusion with 25mM ASA plus 50mM HCl in the presence of clopidogrel (30 mg/kg), in rats without (B) or with pretreatment of egualen (10 mg/kg) (C). Significant difference at P<0.05; * from control (without clopidogrel); # from vehicle. |
Effect of egualen on loxoprofen-induced small intestinal damage
Orally administered loxoprofen (60 mg/kg) in normally fed rats produced multiple hemorrhagic lesions in the small intestine, the lesion score being 223.2±25.1 mm2 (Fig. 6). When the animals were pretreated with egualen (3–30 mg/kg) given p.o. twice daily for 3 days prior to loxoprofen treatment, the development of these intestinal lesions was prevented in a dose-dependent manner, and a significant effect was observed at 30 mg/kg, the inhibition being 64.3%. Likewise, this drug exhibited a prophylatic effect when administered acutely twice 30 min before and 6 hours after the administration of loxoprofen. In this case, egualen (10–100 mg/kg) also dose-dependently reduced the severity of the small intestinal lesions generated by loxoprofen, although the effective dose was slightly higher than that of the repeated administrations for 3 days. In particular, this drug at 100 mg/kg significantly suppressed the occurrence of these lesions in response to loxoprofen; the inhibition being 68.7%. Histologically, the lesions produced by loxoprofen were deep in the mucosa, almost reaching the muscularis mucosae (Fig. 7), while the severity of the damage was markedly lessened in rats pretreated with egualen (100 mg/kg).
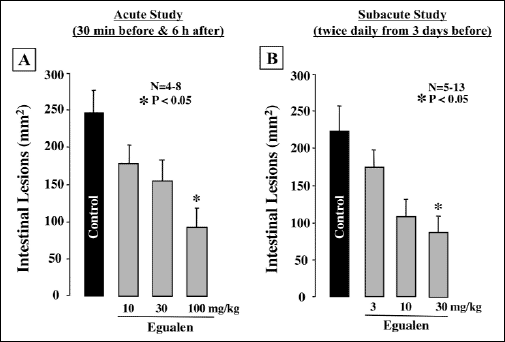 |
Fig. 6. Effect of egualen on loxoprofen-induced small intestinal damage in rats. Animals were given loxoprofen (60 mg/kg) p.o. and killed 24 hours later. Egualen (10–100 mg/kg) was given p.o. twice 30 min before and 6 hours after the administration of loxoprofen or twice daily (9:30 AM and 8:30 pM) for 3 days prior to loxoprofen treatment. Data are presented as the mean±S.E. for 4–13 rats. *Significant difference from control, at P<0.05. |
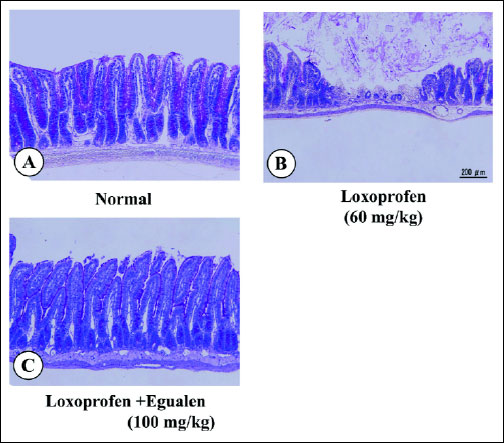 |
Fig. 7. Microscopical observations of small intestinal lesions induced by loxoprofen in rats, with or without pretreatment with egualen. Animals were given loxoprofen (60 mg/kg) p.o. and killed 24 hours later. Egualen (100 mg/kg) was given p.o. twice 30 min before and 6 hours after loxoprofen. Figures show: (A) normal; (B) loxoprofen alone; and (C) egualen plus loxoprofen. Note that loxoprofen caused deep damage in the mucosa, but the severity of damage was apparently lessened by prior administration of egualen. |
Effect of egualen on various events induced in the small intestinal mucosa by loxoprofen treatment
It was found that egualen prevented the occurrence of damage in the small intestine after loxoprofen treatment when the animals were even acutely pretreated 30 min before loxoprofen treatment, and this effect at 100 mg/kg was significant and reproducible. To further investigate the functional mechanism responsible for the prophylactic action of egualen, we examined the effects of egualen at 100 mg/kg on various events that are considered critical in the pathogenesis of NSAID-induced enteropathy (24).
Enterobacterial invasion
Aerobic and anaerobic bacterial counts in the normal intestinal mucosa were 6.11±0.30 and 6.49±0.34 log CFU/g tissue, respectively. Those following the administration of loxoprofen (60 mg/kg) were about 70–90 times greater after 6 hours, being 8.02±0.47 and 8.04±0.40 log CFU/g tissue, respectively. Pretreatment with egualen (100 mg/kg) given p.o. 30 min before loxoprofen significantly suppressed the enhanced invasion of enterobacteria following loxoprofen treatment, the number of bacteria being 6.76±0.26 and 6.88±0.22 log CFU/g tissue, respectively.
Mucosal expression of iNOS mRNA
Expression of iNOS mRNA was not detected in the normal intestine, yet markedly upregulated in the mucosa when examined 6 hours after the administration of loxoprofen (60 mg/kg) (Fig. 8). The upregulation of iNOS expression caused by loxoprofen was mitigated by prior administration of egualen (100 mg/kg).
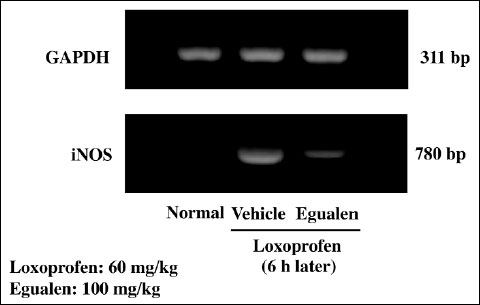 |
Fig. 8. Effect of egualen on the expression of iNOS mRNA in the rat small intestine after the administration of loxoprofen. The animals were given loxoprofen (60 mg/kg) p.o., and the expression of iNOS mRNAs was examined 6 hours later by RT-PCR. Egualen (100 mg/kg) was given p.o. 30 min before the administration of loxoprofen. M: Marker. Note that loxoprofen upregulated iNOS expression, but this response was apparently mitigated by prior administration of egualen. |
Mucus secretion
In the normal intestinal mucosa, PAS-positive substances were clearly observed over the surface epithelial cells and along the glands (Fig. 9A). Loxoprofen (60 mg/kg) apparently reduced the amount of PAS-positive substances on the epithelial cells as well as in the glands (Fig. 9B). However, when the animals were pretreated with egualen (100 mg/kg, p.o.) before the loxoprofen treatment, the decrease in PAS staining was prevented and the amount of PAS-positive materials in the mucosa was largely restored (Fig. 9C). In addition, egualen alone also increased the amount of PAS-positive substances in the mucosa when compared to the control mucosa (Fig. 9D).
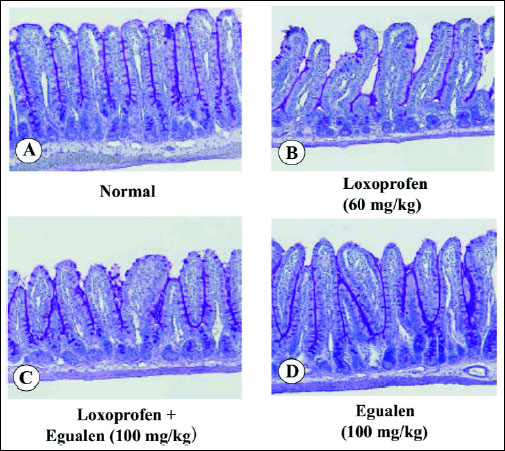 |
Fig. 9. Effect of loxoprofen on mucus secretion (PAS staining) in the rat small intestine, with or without loxoprofen treatment. The animals were given loxoprofen (60 mg/kg) or egualen (100 mg/kg) p.o. and killed 3 hours later for examination of PAS staining. In the combined administration, egualen was given p.o. 30 min before loxoprofen. Figures show: (A) normal; (B) loxoprofen alone; (C) egualen plus loxoprofen; (D) egualen alone. (PAS; x100). Note that loxoprofen markedly decreased PAS-positive materials in the mucosa, but this response was apparently prevented by prior administration of egualen. |
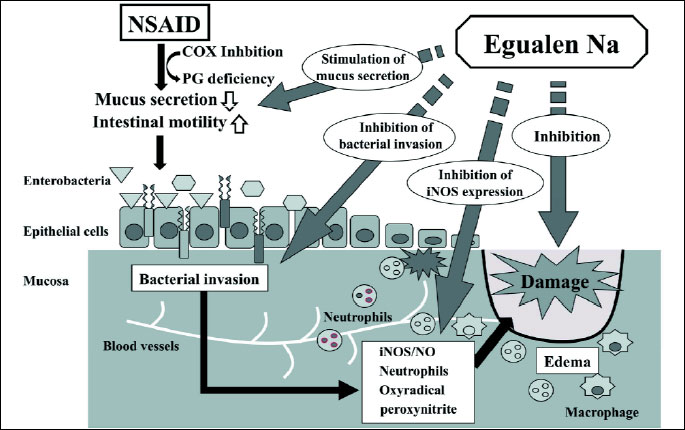 |
| Fig. 10. Factors involved in the development of loxoprofen-induced small intestinal damage, and the influences of egualen on these processes. Loxoprofen causes functional changes such as an increase in intestinal motility and a decrease in mucus secretion, followed by enterobacterial invasion in the mucosa. Endotoxin released from enterobacteria upregulates iNOS expression and NO production as well as inflammation, which results in damage to the small intestine. Egualen increases mucus secretion and hampers the mucosal invasion of enterobacteria, and by so doing suppresses the upregulation of iNOS expression, eventually leading to protection against damage in the small intestine. |
Egualen, a stable azulene derivative, has been used to treat gastritis and gastric ulcers (4, 5). Although previous studies have showed a prophylactic effect of this drug in various models of damage in the stomach and duodenum (1-3, 6), no study had evaluated the effectiveness of egualen on small intestinal damage. In the present study, we examined the effect of egualen on NSAID-induced enteropathy as well as gastric injury induced by I/R or dual antiplatelet therapy, and found that the drug effectively suppressed the occurrence of damage.
First, we examined the effect of egualen on the development of gastric damage under I/R conditions. Previous study showed that I/R-induced gastric damage was worsened by pretreatment with indomethacin and rofecoxib (a selective cyclooxygenase (COX)-2 inhibitor) but not SC-560 (a selective COX-1 inhibitor), and this aggravation was abrogated by co-administration of iloprost (a prostacyclin (PGI2) analogue), suggesting a role for COX-2/PGI2 in mucosal defense under such conditions (8, 26). Furthermore, we have also shown that the severity of these lesions was reduced by pretreatment with ozagrel or seratorodast, similar to iloprost, suggesting the involvement of COX-1/TXA2 in the pathogenesis of I/R-induced gastric damage, in addition to COX-2/PGI2 (9). As expected, the present study showed that egualen significantly prevented the development of gastric damage under I/R conditions, similar to ozagrel and seratorodast. We also confirmed the neutrophil infiltration in the stomach during I/R-treatment, as represented by a marked increase of MPO activity (27), and further observed that egualen attenuated the increase of MPO activity following I/R treatment. Because egualen is known to have a TXA2 antagonistic action (7), the present results further supported the pathogenic importance of TXA2 in I/R-induced gastric damage.
Recent clinical studies showed that the risk of gastric bleeding is increased by the concomitant use of antiplatelet drugs with NSAIDs or low-dose ASA in the presence of exogenous or endogenous acid in the rat stomach (15, 16, 28). Clopidogrel is an antiplatelet drug that specifically and irreversibly inhibits the P2Y12 subtype of the adenosine diphosphate receptor, which is important to the aggregation of platelets and cross-linking by the protein fibrin (29). The present study confirmed in rats that pretreatment with clopidogrel aggravated gastric bleeding in response to acidified ASA, and eventually increased the severity of the acidified ASA-induced hemorrhagic damage in the stomach. Ritchie et al. (30) proposed three factors essential for the generation of gastric lesions; 1) gastric barrier disruption, 2) luminal acid, and 3) gastric mucosal ischemia. ASA is known to disrupt the gastric mucosal barrier, damage the stomach in the presence of acid, and attenuate the gastric hyperemic response to acid back-diffusion following barrier disruption by suppressing PG production (31). Thus, the model we used fulfills the conditions necessary to generate damage in the stomach. In the present study, egualen dose-dependently and significantly mitigated gastric bleeding and ulcerogenic responses to acidified ASA plus clopidogrel and the effect was almost equivalent to that of PGE2. Similar effects have been observed with several other agents used as mucosal protective drugs in the treatment of gastric ulcers (13, 14, 28). The mechanism by which egualen prevented gastric bleeding and damage under these conditions remains unknown. However, because egualen binds to the gastric mucosa via a non-specific hydrophobic interaction to form a complex that is less vulnerable to ASA irritation and because this drug exhibits gastric hyperemic and anti-inflammatory effects (1-3, 6, 7), it is assumed that egualen prevents gastric bleeding and lesions under such conditions, probably by its local mucosal protective action and anti-inflammatory action. At present, the clinical effectiveness of mucosal protective drugs against gastric bleeding associated with dual antiplatelet therapy remains unproven. Further animal and clinical studies are needed to clarify these points.
Most important in the present study is the finding that egualen, given acutely or subacutely for 3 days before, prevented the development of small intestinal damage following the administration of loxoprofen, a NSAID frequently used in Asian countries. NSAIDs cause intestinal ulceration in humans and laboratory animals after short-term and long-term administration (32, 33). In particular, recent clinical studies using capsule endoscopes or double-balloon endoscopes confirmed that NSAIDs damage the small intestine at a higher incidence than previously thought (34). Several factors have been implicated in the pathogenesis of NSAID-induced small intestinal ulceration, including bacterial flora, bile acid, and hypermotility, in addition to PG deficiency (24, 25, 35). Since egualen did not affect the decreased PGE2 content in the presence of loxoprofen (data not shown), it is assumed to act downstream of the events resulting from the PG deficiency caused by loxoprofen and eventually prevent the development of small intestinal lesions.
NSAID-induced intestinal damage is prevented by pretreatment with antibiotics such as ampicillin (24, 25), suggesting a key pathogenic role for enterobacteria in this model. The importance of bacterial was also demonstrated in the pathogenesis of not only the small intestinal damage but also colonic damage induced by NSAIDs (36). Boughton-Smith et al. (37) reported that bacterial endotoxin enhanced intestinal permeability through up-regulation of iNOS expression and overproduction of NO in the mucosa. This was further supported by the findings that indomethacin increased iNOS activity and NO production, preceeding the onset of intestinal damage, and that aminoguanidine prevented the intestinal ulcerogenic response by suppressing NO production due to iNOS (24). As expected, we observed that loxoprofen caused bacterial invasion in the mucosa, followed by the up-regulation of iNOS expression and MPO activity, and these responses were suppressed by egualen. These findings suggest that the protective effect of egualen against loxoprofen- induced intestinal damage is functionally associated with the down-regulation of iNOS expression resulting from the suppression of bacterial invasion. The mechanism by which enterobacteria invade the mucosa remains unknown, yet previous studies suggest that a decrease in mucus secretion and an increase of mucosal permeability may contribute to this process after indomethacin treatment (22, 38, 39). Since mucus plays a crucial role in innate host defenses against intestinal pathogens and irritants, it is possible that a decrease in mucus secretion weakens the intestinal barrier, resulting in bacterial invasion.
In the present study, we found that the amount of PAS-positive materials in the small intestine was markedly reduced after loxoprofen treatment, but this response was restored by prior administration of egualen. Furthermore, egualen by itself apparently increased the amount of PAS-positive substances in the mucosa. It is assumed that egualen stimulates mucus secretion, thereby increasing the mucus gel’s thickness and hampering bacterial invasion following the administration of loxoprofen. Thus, the present results together suggest that egualen protects the small intestine against loxoprofen-induced damage, and this effect may be functionally associated with an increase in the secretion of mucus, resulting in suppression of bacterial invasion and iNOS expression, the major pathogenic events in NSAID-induced small intestinal ulceration (Fig. 10).
Given the findings of the present study, we conclude that egualen has a prophylactic effect against gastric damage induced by I/R and gastric bleeding induced by double antiplatelet therapy with ASA plus clopidogrel as well as small intestinal damage generated by loxoprofen, probably through its characteristic pharmacological properties, such as TXA2 antagonistic action, local mucosal protection, and increase in mucosal blood flow as well as mucus secretion (2, 3, 7).
Acknowledgements: This work in its preliminary from has been presented during 7th International Symposium on Cell/Tissue Injury and Cytoprotection Organoprotection. September 9-11 2012, Honolulu, Hawaii organized by Prof. K. Takeuchi (Kyoto, Japan) and Prof. H. Matsui (Tsukuba, Japan).
Conflict of interests: None declared.
- Okabe S, Takeuchi K, Mori Y, Furukawa O, Yamada Y. Effects of KT1-32 on gastric lesions and duodenal ulcers induced in rats. Nihon Yakurigaku Zasshi 1986; 88: 467-476.
- Yano S, Horie S, Wakabayashi S, Mochizuki S, Tomiyama A, Watanabe K. Increasing effect of sodium 3-ethyl-7-isopropyl-1-azulenesulfonate 1/3 hydrate (KT1-32), a novel antiulcer agent, on gastric mucosal blood flow in anesthetized rats. Res Commun Chem Pathol Pharmacol 1990; 70: 253-256.
- Mochizuki S, Matsumoto M, Wakabayashi S, Kosakai K, Tomiyama A, Kishimoto S. Therapeutic effect of egualen sodium (KT1-32), a new antiulcer agent, on chronic gastritis induced by sodium taurocholate in rats. J Gastroenterol 1996; 31: 785-792.
- Miyoshi A, Okabe H, Miwa T, et al. Clinical evaluation of egualen sodium in the treatment of gastric ulcer: a multicenter double-blind comparative study with cetraxate hydrocholoride. Jpn Pharmacol Ther 1999; 27: 837-852.
- Miyoshi A, Miwa T, Nakazawa S, Kajiyama G, Hayakawa A, Ogawa N. Combined therapy with egualen sodium and cimetidine for patients with gastric ulcer: clinical evaluation of initial treatment. A multicenter controlled study in comparison with cometidine alone. Jpn Pharmacol Ther 1999; 27: 853-868.
- Rogers C, Brown A, Szabo S. Gastric mucosal protection by new aryl sulfhydryl drugs. Dig Dis Sci 1988; 33: 324-329.
- Mochizuki S, Wakabayashi S, Tomiyama A, Satake N, Shibata S. Thromboxane A2 antagonistic action of a new anti-ulcer agent, azuletil sodium (KT1-32). Scan J Gastroenterol 1989; 24: 194-197.
- Kotani T, Kobata A, Nakamura E, Amagase K, Takeuchi K. Roles of COX-2 and prostacyclin/IP receptors in mucosal defense against ischemia/reperfusion injury in mouse stomach. J Pharmacol Exp Ther 2006; 316: 547-555.
- Takeuchi K, Kojima S, Abe N, Takayama S. Pathogenic importance of thromboxane A2 in ischemia/reperfusion-induced gastric injury in mice. (abstract). Gastroenterology 2011; 140 (5 Suppl. 1): S-314.1.
- Hori Y, Katori M, Harada Y, Uchida Y, Tanaka K. Potentiation of bradykinin-induced nociceptive response by arachidonate metabolites in dogs. Jpn J Pharmacol 1986; 132: 47-52.
- Camposo D, Cataldo MG, Paterna S, Bivona A, Barbarino C. Cytoprotective therapy of gastric ulcers: a controlled clinical evaluation of triletide versus carbenoxolone. Pharmatherapeutica 1985; 4: 166-170.
- Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest 2004; 113: 340-345.
- Izuhara C, Yamasaki K, Honda M, Nishio H, Takeuchi K. Aggravation by clopidogrel, an anti-platelet drug, of HCl/aspirin-induced gastric bleeding and lesions in rats: prophylactic effect of rebamipide. Jpn Pharmacol Ther 2010; 38: 333-345.
- Izuhara C, Takayama S, Itayama M, Hoshin N, Yoshida Y, Takeuchi K. Animal model for evaluating gastric bleeding and ulcerogenic response induced by aspirin plus clopidogrel. In: Cell/Tissue Injury and Cytoprotection/Organoprotection in the Gastrointestinal Tract: Mechanism, Prevention and Treatment, L. Filaretova, K. Takeuchi K, (eds). Karger Publication 2012; pp. 87-96.
- Hallas J, Dall M, Andries A, et al. Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: population based case-control study. Brit Med J 2006; 333: 726.
- Uchiyama S, Yamazaki M, Nakamura T, et al: New modalities and aspects of antiplatelet therapy for stroke prevention. Cerebrovasc Dis 2006; 21(Suppl. 1): 7-16.
- Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG.. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol 2005; 3: 133-141.
- Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology 2005; 128: 1172-1178.
- Fujimori S, Seo T, Gudis K, et al. Prevention of nonsteroidal anti-inflammatory drug-induced small-intestinal injury by prostaglandin: a pilot randomized controlled trial evaluated by capsule endoscopy. Gastrointest Endosc 2009; 69: 1339-1346.
- Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity: assessment of inflammation in rat and hamster models. Gastroenterology 1984; 87: 1344-1350.
- Holzer P, Pabst MA, Lippe IT. Intragastric capsaicin protects against aspirin-induced lesion formation and bleeding in the rat gastric mucosa. Gastroenterology 1986; 96: 1425-1433.
- Amagase K, Ochi A, Sugihara T, Kato S, Takeuchi K. Protective effect of lafutidine, a histamine H2 receptor antagonist, against loxoprofen-induced small intestinal lesions in rats. J Gastroenterol Hepatol 2010; 25: S111-S118.
- Deitch EA, Ma L, Ma WJ, et al. Inhibition of endotoxin-induced bacterial translocation in mice. J Clin Invest 1989; 84: 36-42.
- Takeuchi K, Yokota A, Taniguchi M, Takahira Y, Tanaka A. Factors involved in up-regulation of inducible nitric oxide synthase in rat small intestine following administration of nonsteroidal antiinflammatory drugs. Dig Dis Sci 2006; 51: 1250-1259.
- Takeuchi K, Tanaka A, Kato S, Amagase K, Satoh H. Roles of COX inhibition in pathogenesis of NSAID-induced small intestinal damage. Clinica Chimica Acta 2010; 411: 459-466.
- Nakamori Y, Komatsu Y, Kotani T, Kojima S, Takeuchi K. Pathogenic importance of cysteinyl leukotrienes in development of gastric lesions induced by ischemia/reperfusion in mice. J Pharmacol Exp Ther 2010; 333: 91-98.
- Kobata A, Kotani T, Komatsu Y, Amagase K, Kato S, Takeuchi K. Dual action of nitric oxide in the pathogenesis of ischemia/reperfusion-induced mucosal injury in mouse stomach. Digestion 2007; 75: 188-197.
- Takayama S, Izuhara C, Yamada N, et al. A new model of gastric bleeding induced in rats by aspirin plus clopidogrel under stimulation of acid secretion: prophylactic effects of antiulcer drugs. J Physiol Pharmacol 2012; 63: 41-52.
- Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest 2004; 113: 340-345.
- Ritchie WP. Acute gastric mucosal damage induced by bile salts, acid, and ischemia. Gastroenterology 1975; 68: 699-707.
- Takeuchi K, Ueki S, Tanaka H. Endogenous prostaglandins in gastric alkaline response in the rat stomach after damage. Am J Physiol 1986; 250: G842-G849.
- Bjarnason I, Zanelli G, Smith T, et al. Nonsteroidal anit-inflammatory drug-induced intestinal inflammation in humans. Gastroenterology 1987; 93: 480-489.
- Laine L. GI risk and risk factors of NSAIDs. J Cardivasc Pharmacol 2006; 47(Suppl. 1): S60-S66.
- Kameda N, Higuchi K, Shiba M, et al. A prospective, single-blind trial comparing wireless capsule endoscopy and double- balloon enteroscopy in patients with obscure gastrointestinal bleeding. J Gastroenterol 2008; 43: 434-440.
- Takeuchi K, Miyazawa T, Tanaka A, Kato S, Kunikata T. Pathogenic importance of intestinal hypermotility in NSAID-induced small intestinal damage in rats. Digestion 2002; 66: 30-41.
- Zwolinska-Wcislo M, Krzysiek-Maczka G, Ptak-Belowska A, et al. Antibiotic treatment with ampicillin accelerates the healing of colonic damage impaired by aspirin and coxib in the experimental colitis: importance of intestinal bacteria, colonic microcirculation and proinflammatory cytokines. J Physiol Pharmacol 2011; 62: 357-368.
- Boughton-Smith NK, Evans SM, Laszlo F, Whitle BJ, Moncada S. The induction of nitric oxide synthase and intestinal vascular permeability by endotoxin in the rat. Br J Pharmacol 1993; 110: 1189-1195.
- Kamei K, Kubo Y, Kato N, Hatazawa R, Amagase K, Takeuchi K. Prophylactic effect of irsogladine maleate against indomethacin-induced small intestinal lesions in rats. Dig Dis Sci 2008; 53: 2657-2666.
- Amagase K, Ochi A, Kojo A, et al. Prophylactic effect of monosodium glutamate against NSAID-induced enteropathy. J Pharmacol Sci 2012; 118: 131-137.