ACRYLAMIDE-INDUCED PRENATAL PROGRAMMING OF INTESTINE
STRUCTURE IN GUINEA PIG
INTRODUCTION
A complex of maternal, placental and fetal factors is involved in ensuring normal foetal growth. The structural quality of new formed intestinal tract is determined mainly by genes, and fluctuates with age and health being under the influence of hormonal and nutritional modification during prenatal and postnatal time in both animals and humans. Nutrition plays a main role in the early structural development of mammals and has long-term effects that are evident until later in life (1, 2). Foetal programming refers to the process by which an acute or chronic stimulus in utero establishes a permanent response in the foetus that impacts physiological function in later life (2-5).
Acrylamide (ACR) is a water-soluble, vinyl monomer used in preparing polymers and copolymers containing polar functional groups. Besides the industrial and laboratory uses the general population is exposed to varying amounts of ACR <i>via</i> the diet. It is formed in foods upon heating as a consequence of asparagine degradation in the presence of reducing sugars as well as carbonylic compounds deriving from either the Maillard reaction or lipid oxidation processes (6). ACR is rapidly absorbed after oral or dermal contact. Exposure to ACR results in a peripheral neuropathy, with accompanying weakness of the limbs in humans and animals (7). It is also considered a reproductive and developmental toxin, with mutagenic and carcinogenic properties in experimental mammalian in vitro and in vivo systems (8). Testing of laboratory animals showed also an increase of tumors in many organs including intestinal tract (9-12).
The dietary intake of ACR is difficult to assess because of the lack of information on ACR level in many products and food processing procedures, especially for home made meals. The WHO estimated that the average dietary ACR exposure for the general population ranges between 0.3 and 0.8 µg/kg of body weight per day (13).
Most of our knowledge about the physiology and pathology of foetal growth is derived from many laboratory animal studies. However, basal information about their gastrointestinal tract development is not completely known. Significant inter-species differences were observed in the anatomy and physiology of the gut (pH profile, water content and distribution/number of lymphoid follicles) (14). However, the authors consider that the guinea pig used in this study may be suitable for specific studies related to the gastrointestinal tract because the guinea pig has a high degree of neurological and neuroendocrine maturity at birth, and most rapid organs growth occurs around middle of gestation terms 68 days. The development of intestinal tract takes place during prenatal time and female guinea pig gives birth to mature offspring. Therefore, in the present study guinea pigs were examined as a most suitable among rodents to nutritional maternal programming. Moreover, little is known about a role of ACR in prenatal programming of postnatal development of gastrointestinal tract assessed on the basis of the morphometry and expression of the one of proteins in adherent type cell-cell junctions in small intestine epithelium.
Available data relate to other organs like spinal cord, medulla oblongata or liver of offspring obtained from pregnant rats treated with ACR at the dose of 10 mg/kg/day, and ovaries of guinea pigs, which mothers were treated with ACR at the dose of 3 mg/kg/day (8, 15-17).
The current study examined the effect of ACR-induced programming on histomorphometric parameters in target tissues of ACR induced tumors - intestine at daily dose of 3 mg/kg/day.
MATERIALS AND METHODS
The experimental procedures used throughout this study were approved by The Local Ethics Committee on Animal Experimentation of University of Life Sciences in Lublin, Poland.
Pregnant guinea pigs
Ten randomly chosen primiparous female guinea pigs (Himalayan-Guinea-Pig), about 420 g of body weight each, and their litters were used in this experiment. Animals were purchased from the Institute of Microbiology and Immunology at Lodz University. The female guinea pigs were 90 days old upon arrival, mated naturally using a technique of IV-block matching system of group - breeding outbred with healthy fertile males (at a ratio of 1 male to 2 females). This method produces accurately time pregnant guinea pigs. Vaginal plugs were examined. Vaginal smear examination was carried out to provide a precise determination of the onset of gestation.
Guinea pigs were clinically healthy and singly housed in standardized boxes for a guinea pig, type Velaz providing them sufficient space, appropriate equipment and possibility of social contacts. The temperature was 20 ±–2°C, humidity 55–60%, controlled 12:12-h light-dark cycle with airflow speed does not exceed 0.3 m/s. Guinea pigs had free access to fresh water and solid food. Energy needs of feed was based on the nutrient requirements of laboratory animals (18). The feed was weighed daily and the food consumption was established. Experiment started at 32 days of gestation (term, approximately 67 days of gestation). The gestation length did not differ between control and experimental females. Animals were divided in two groups depending on diet supplementation and weighed every week. Five guinea pigs (ACR-mothers) received acrylamide during the last 35 days of pregnancy, while the other five were control (C-mothers). The body weight gain of pregnant guinea pigs from control and Ac groups did not differ. Mean number of stillborn and live born offspring in litters from Ac- and C-mothers also did not differ. None of the pregnant females exhibited signs of toxicity, discomfort or behavioural anomalies, sickness or mortality during the study period.
Preparation and analysis of drinking water and food consumption
It was found suitable to use the commercial ACR for electrophoresis in the form of powder (minimum 99%) (Sigma-Aldrich). Diluted or powdered ACR was kept in a freezer at –20°C.
Water consumption during 24 hour, for 2 groups of pregnant guinea pigs, was measured before the beginning of the experiment. The water or ACR water solution were changed daily. The water volume left in the bottles was measured and data were put in a table to follow the consumption of ACR through the water. The dose applied was selected on the basis of literature so as not to induce characteristic sign of acrylamide-induced neurotoxicity, such as hindlimb foot splaying or serious foetal abnormalities. However, this dose is used in animal experiments and is considered to be low (7, 19).
These data, combined with body weight, were used to calculate and maintain the amount of ACR of 3 mg/kg b.w./day given in tap water. There were no differences in water consumption between the groups. Food consumption was measured daily in control animals and treated with ACR and there was no differences.
Newborns
Normal litter size is two to five offspring. Females and males from each litter were treated in the same manner, weighed and used in subsequent study for intestine analysis. All newborns born naturally had no congenital infections. The incidence of growth abnormalities of morphologic defects was not observed. None of pups exhibited signs of toxicity, discomfort or behavioural anomalies, sickness or mortality during the study period. In this model, randomly chosen and clinically healthy pups were redistributed shortly after the birth to either control (n=6, born by C-mothers) or supplemented group (n=6, born by ACR-mothers) and euthanized one by one with CO2 inhalation and by dislocation of the spine. Tissue samples were taken from each sacrificed animal.
Tissue collection and histomorphometry analysis
Two, 10 mm long, segments of the small intestine, one from the duodenum (10 mm distal to the pylorus) and second from 50% of the total intestinal length (representing jejunum), were taken from each animal. The tissue samples were subjected to histology as described previously, except the sections were cut at 4 m thick (2, 11). Masson's trichrome staining was used to differentiate the small intestine wall layers more efficiently (20). Hoechst and eosin staining was used to count apoptotic cells as described previously (11). Moreover, picro sirius red staining (PSR) and polarization microscopy were used to identify the distribution of thick and thin collagen fibres. Thicker collagen fiber (type I or mature collagen) had red color, and thinner fibre (type III or immature collagen) had green color (21, 22). All tissue handling was done by the use of the same equipment as described previously (2, 11).
Microscopic (two-dimensional) images were collected using a confocal microscope (AXIOVERT 200M equipped with an LSM Pascal 5 scanning head; Carl Zeiss, Jena, Germany) with an argon laser (514 nm). Images were combined from two channels: laser scans and the Nomarski technique. AxioCam HRc camera (Carl Zeiss, Jena, Germany) was also used for image acquisition. Objective magnifications of 4×, 10×, 20× and 40× were used to show the different intestinal structures and to collect images of the examined tissues from each specimen for further analysis (2, 11). The structure of the small intestine wall was examined under microscopic observation and with the use of graphic analysis software (ImageJ 1.48b, National Institute of Health, Bethesda, MD, USA; available at: http:\\rsb.info.nih.gov/ij/index.html).
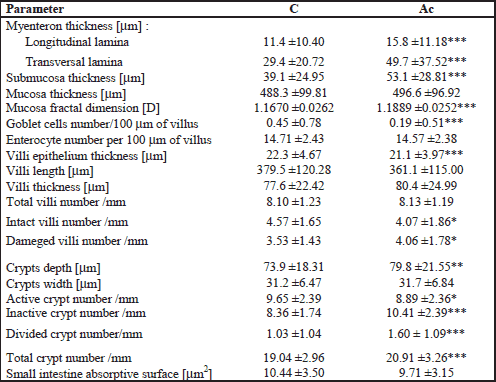
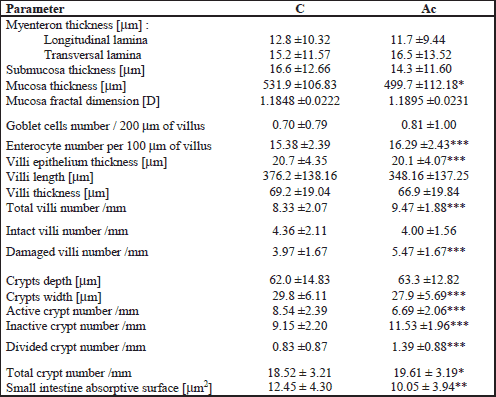
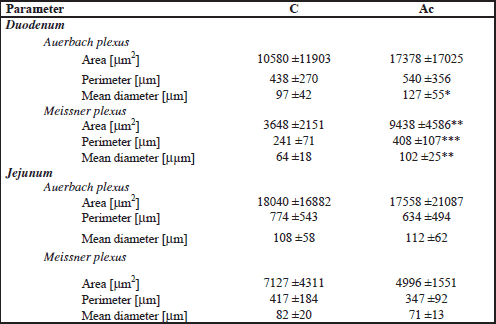
The following morphometric variables were analyzed: mucosa, submucosa and myenteron (longitudinal and transversal lamina) thickness; mucosa fractal dimension (according to the box-counting method (23), goblet cells and enterocyte number per 100 m of villi, villi epithelium thickness, crypt depth (defined as the depth of the invagination between adjacent villi, from the bottom of the crypt to the base of villi); crypts width (measured in the middle of crypt depth); the crypts number (active: showing mitoses and Paneth cells, having an open internal space and access to the intestinal lumen; inactive: showing no mitoses and Paneth cells, having a closed internal space; divided: fission crypts as described previously (24); total: active plus inactive and divided crypts); villi length (from the tip of the villi to the villous-crypt junction); villi thickness (measured in the middle of villi height); the villi number (intact and damaged); small intestine absorptive surface (25). Only vertically oriented villi and crypts were measured. Nerve ganglia of Auerbach and Meissner plexuses were measured regarding its area, perimeter and mean diameter.
Apoptotic cells were counted per square millimetre of tissue (in Hoechst eosin-stained sections); the criteria for recognizing apoptotic cells were those outlined by Lizard et al. (26) and Majno and Joris (27).
Immunohistochemistry
Immunohistochemical staining was performed according to protocol described previously (2, 11). Mouse monoclonal to pan Cadherin antibody (Abcam, Cambridge, UK, dilution 1:200) was used as a first antibody to mark adherent type cell-cell junctions in small intestine epithelium. Biotinylated anti-rat immunnoglobulin (DacoCytomation, Glostrup, Denmark, dilution 1:200) was used as a second antibody. Negative control sections from each animal received identical preparations for immunohistochemical staining, except that the primary antibody was omitted. Microscopic observations and images of immunohistochemistry reactions were further analyzed. The expression of cadherin was described as the intensity (low, high or higher) and type of immunohistochemical reaction (the width of the reaction).
Statistical analysis
All results are expressed as means ±S.D. (standard deviation). Differences between means were tested with the Student-T test. Normal distribution of data was examined using the W. Shapiro-Wilk test and equality of variance tested by the Brown-Forsythe test. When there was a lack of normal distribution and/or unequal variance of data, we had to use of the Mann-Whitney U test to test the differences between means. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were carried out by means of STATISTICA data analysis software, version 8.0. (Stat Soft, Inc.; 2008).
RESULTS
Gastrointestinal tract morphology
The histomorphometric analysis of the duodenum and jejunum, and nerve plexuses is presented in Table 1, 2 and 3, respectively. The thickness of myenteron and submucosa, mucosa fractal dimension and the depth of crypts in the duodenum were increased by ACR (Figs. 1a and 1b). Moreover, maternal ACR treatment increased the number of total, divided and inactive crypt, and the number of damaged villi number in the duodenum and jejunum of newborn guinea pigs. Additionally, ACR increased the total villi number in the jejunum. In offspring born by mothers administered with ACR the decrease of villi epithelium thickness and active crypt number was observed. Moreover, ACR decreased goblet cells number and inact villi number in the duodenum and mucosa thickness and crypts width in the jejunum (Figs. 1c and 1d). Intestine absorptive surface was affected by ACR in the jejunum as well.
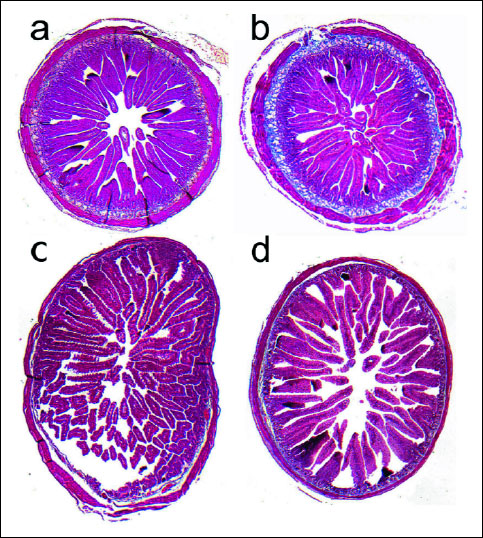 |
Fig. 1. Light micrographs of duodenum wall of (a) - control and (b) - maternal ACR treated newborn guinea pigs, demonstrating the increase of myenteron and submucosa thickness; and (c) - jejunum wall of control and (d) - ACR treated newborn guinea pigs, showing the increase of mucosa thickness. |
Additionally, ACR influenced the size of nerve plexuses in duodenum, but stronger influence was observed in Meissner plexus.
Immunohistochemistry
The immune reaction with anti-cadherin antibodies in the duodenum and middle part of the jejunum in the control group of offspring showed higher and continuous expression (staining intensity was strong). The expression for cadherin in the Ac group was lower and distracted (Fig. 2).
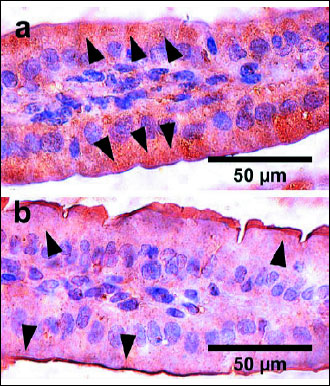 |
Fig. 2. Representative pictures of the immunohistichemical reactions of pan Cadherin carried out on formaldehyde-fixed sections from the duodenum of clinically healthy newborn guinea pig from the control group - no treatment with acrylamide (a), the ACR group - being under the influence of acrylamide for the last 35 days of prenatal life (b). Examples of the reaction are marked with black arrows. |
Apoptosis in small intestine
Apoptotic cell number was significantly increased by maternal ACR administration. Apoptotic cell number counted for duodenal mucosa (per square millimetre) reached the value of 5.52 ± 1.38 in the control group, and 4.47 ± 0.84 in the ACR group. While, in mucosa of jejunum reached the value of 3.18 ± 0.69 and 5.84 ± 0.81 for the control and ACR group (P<0.05), respectively. Apoptotic cell number counted for total area of cross section of jejunum also increased significantly in the ACR group compared with the control group and reached the value of 3.24 ± 0.72 and 5.64 ± 0.37 for the control and ACR group (P<0.05), respectively. Apoptotic cell number counted for total duodenal cross section reached the value of 5.03 ±0.98 in the control group, and 4.03 ± 0.85 in the ACR group.
Collagen fibres
PSR staining was evaluated and cross-polarization microscopy was used to identify the collagen total area, and qualify the type I (mature) and type III collagen (immature). Because the thick collagen had red and thin collagen had green color, it was possible to determine the are occupied by the total collagen. At birth, animals in ACR group showed a predominance of type I collagen and the increase of the collagen total area (submucosa thickness) in duodenum (Figs. 3a and 3b), and the decrease of mature and the increase of immature collagen in jejunum (Figs. 3c and 3d).
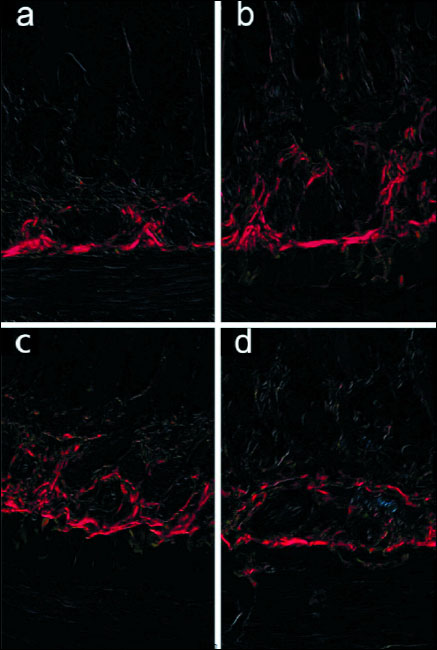 |
Fig. 3. Representative photographs of the collagen total area. Collagen type I - mature (red) and collagen type III - immature (green) in duodenum of (a) - control; (b) - maternal ACR treated newborns; and in jejunum of (c) - control; and (d) maternal ACR treated newborns. The 4 µm sections of full-thickness intestine were PSR stained. |
DISCUSSION
ACR formation in foods has received increased worldwide attention in recent years because of the potential toxicity of this product (28). ACR is rapidly and extensively absorbed by many routes including oral route and it or its metabolites bind to DNA, RNA and protein.
To our knowledge, the neurotoxicity or genotoxicity of ACR is very complex. In addition, the metabolism and distribution of ACR was intensively studied in rodents and other animals at daily dosage of 0.5–50 mg/kg/day (16, 17). In animal studies, ACR has been shown to increase the incidence of tumors in several glands: thyroid, mammary, adrenal, pituitary. It is clear that tumors occur following direct damage to the hormone-producing organ (29). However, little is known about a role of ACR-induced prenatal programming. Moreover, limited observation in humans should be supported by the information obtained in animals studies showing that the pattern of intrauterine growth influences postnatal function of the intestinal tract and skeletal system (4, 30-33). The exposure to ACR in animal pregnancy at the dose, that does not to induce characteristic sign of acrylamide-induced neurotoxicity, and that is associated with an increased risk of bowel malformation was not demonstrated and examined previously.
In April 2002 the Swedish National Food Administration and researchers from Stockholm University first reported the presence of elevated levels of ACR in certain types of commonly consumed foods, primarily potato and cereal products that are subject to thermal processing (34). It was reported that more than one-third of the calories consumed by U.S. and European populations contain ACR (35, 36). Also the mean dietary intake of ACR from coffee was estimated to be 12 µg day-1, which is about 39% of the total dietary intake of 31 µg day-1 (37). Roasted coffee is a significant source of dietary ACR intake, supply the total Polish population (1–96 years) 19% of ACR, with the highest amount in the adult population about 27% of total dietary ACR intake (38).
The dose of ACR used in this study for pregnant guinea pigs was corresponded to a daily dose of 340 µg/kg body weight per day for humans, that seems to be a high. But, the mean content of acrylamide in tested 225 samples of foodstuffs taken randomly all over Poland, ranged widely from 11 to 3647 µg/kg of product (38). First of all, the study performed in humans with oral exposure of ACR at the dose of 0.5, 1.0 and 3.0 mg/kg body weight shows basic differences in metabolism and toxicokinetics between rodents and humans, and lower risk for cancer initiated by ACR metabolites in humans, but not pregnant (39).
Because, ACR crosses the placental barrier in humans and animals as well, maternal exposure is a relevant measure of the foetal exposure to ACR (10, 12). Therefore we found that it is interesting to analyze whether ACR in maternal diet can program offspring development because of different manipulations in prenatal development can program the growth of mammals' offspring and modify postnatal development by the change of gut structure and function influencing nutrient and energy absorption in the young animals (2, 40, 41). Moreover, it is important, that ACR is rapidly absorbed to high degree in the intestine (42).
Current results of measurements showed that maternal ACR treatment had negative influence on small intestine histomorphometry associated probably with physiological functions (Table 1 and 2) which in turn may turn out as predominant source of small intestinal lesions as earlier (11, 41). This study suggested that ACR acting prenatally influenced small intestine nervous plexuses (Table 3) that became enlarged by 2.5 times compared with the control group. The perimeter and mean diameter of Meissner plexuses in duodenum increased about 70% and 60%, respectively. Moreover, similar increase in Auerbach plexuses was observed, although not all the changes were statistically significant. But, ACR administered to pregnant guinea pigs increasing the size of nerve plexuses in the duodenum caused the increase of wall thickness. Moreover, transversal lamina of myenteron enhanced as well (by 69%) (Fig. 1). It may result in improper motor activity and secretory function and influence adversely processes of the digestion later in life. On the other hand prenatal administration of ACR to guinea pig resulted in the tendency to the reduction of the size of nerve plexuses and the thinning of intestinal wall in the jejunum (by 6%).
Our earlier study conducted on growing mice shows similar negative impact of ACR on innervation of small intestine wall and indirectly on absorptive function of small intestine mucosa (11).
Maternal ACR treatment decreased the villi epithelium thickness (by 5.5%). It may influence the activity of enterocytes. Additionally, goblet cell number in duodenum of guinea pigs offspring decreased by 57.7%. Further, the decrease of intact villi number (11%) and the increase damaged villi number (15%) was observed. These changes also led to the decrease of small intestine absorptive surface. ACR given to foetuses <i>via</i> their mothers caused the increase of damaged villi number linked with enhanced complexity of duodenal mucosa. Additionally, ACR enhanced the enterocyte number (by 6%) in the jejunum as a compensation of absorptive function.
Our earlier findings show that feed supplementation with ACR affect crypt region (11). Data concerning the influence of maternal ACR administration on intestinal morphology at the birth are limited.
This study also indicated that maternal diet supplemented with ACR influenced the morphology of the most metabolically active region in the intestinal mucosa region - the crypts. The profile of crypt became different from that observed in the control. The decrease of active crypt number (in duodenum by 8%, in jejunum by 21.6%) while the rise of the total (an average of 6%) and inactive crypt number (an average of 25%) in offspring intestine was observed. Reported alteration of selected morphological parameters might indicate that ACR affected its activity. Likely to keep the secretory function of crypts at optimal level other parameters like the depth (by 8%) were increased.
Our earlier study also show that ACR given to mice led to similar changes, although it was given during postnatal time (11). Other study on the effect of ACR on the histopathology of various organs in pregnant mice shows that regular consumption of ACR during pregnancy may cause foatal prenatal and/or postnatal abnormalities and malformation. However livers, kidney, hearts and femora were analyzed (44). Foetus is exposed to ACR through transplacental transfer or glycidamide after maternal metabolization of ACR (10). Glycidamide, the metabolite of ACR is assumed to be the predominant genotoxic agent in ACR exposure. But, as mentioned above, metabolism of ACR differs between rodents and humans (44, 45).
No studies conducted so far included a detailed morphological analysis of the small intestine of offspring born by the guinea pig mothers administered with ACR during weeks 5 to 10 of gestation. In the current paper, authors showed for the first time that prenatal exposure to ACR led to disruption of cellular connections in guinea pig offspring. This study showed that maternal treatment with ACR decreased and limited the expression of cadherin in the epithelium (Fig. 2). It might contribute to the development of cancer in still growing animals.
It is important to recognize that the epithelium of the gut is not composed from functionally identical cells. It is well known that cell-cell interaction plays a key role during development of tissue. Additionally, the cadherins mediate cell adhesion and participate in the maintenance of proper cell-cell contacts (46).
It was mentioned above, that ACR has toxic and teratogenic properties and it is known that tumors occur following direct damage to the hormone-producing organ, but knowledge about its possibility to affect development is limited. Here, it was shown that ACR-induced toxicity and teratogenicity might be primarily based on subsequent induction of apoptosis in intestinal tract at birth.
Our findings showed that acrylamide had a negative influence on small intestinal regeneration and the histomorphometric parameters associated with the physiologic functions of this organ. Probably, more detailed analysis of the main fibrous collagen (type I and III) might show that the healing process also can be disturbed. Based on the data mentioned above, it was speculated that ACR disturbed the intestine development. This finding might indicate that maternal ACR treatment influence not only postnatal development of intestine but finally postnatal general development of offspring. It should be pointed, that ACR was given through the last 35 days of pregnancy in guinea pig. It should be considered what is happening with human offspring development, when pregnant diet contains ACR, even in the quantity deemed acceptable. For this reason further studies with ACR-induced prenatal programming should be conducted to establish if these changes might be permanent and persist throughout the entire period of postnatal development until adulthood. Further, the next step of this study should be design some dose-response experiment (2, 3 mg/kg body weight/day).
It is important to investigate the possible impact of dietary ACR exposure during pregnancy (47). Limited observation in humans should be supported by the information obtained in animals studies. Furthermore, the identification of individuals with high exposure is more important than precise quantitative estimation of ACR in the common diet, because of the assessment of the dietary intake of ACR is difficult, especially for home made meals. This problem should be discussed and pregnant should be educated about this problem. Because, adding every one dose of ACR from food, smoking, drinking water, from dermal contact with products containing residual ACR including cosmetic products, an average daily intake can be higher.
This study showed, that the dose, which did not give clinical signs of neurotoxicity in newborn guinea pig, crossed the placenta and reached the foetus organ in sufficient concentration to disturb and cause an effect in rodents.
Knowledge of the sensitive period for target organ development and the interpretation of optimal data for animals and humans are still limited. The exposure to genotoxic factors in the gestation when foetus rapidly grows and matures give physiologic effects dependent on the dose, frequency and duration of the exposure. Effect observed in exposed individuals may differ because of their genotypically determined differences in metabolism and cell sensitivity.
In conclusion, presented results showed the negative impact of maternal ACR treatment on histological structure, integrity and innervation of small intestine wall as well as on absorptive function of small intestine mucosa.
Conflict of interests: None declared.
REFERENCES
- Dauncey MJ, Bicknell RJ. Nutrition and neurodevelopment: mechanisms of developmental dysfunction and disease in later life. Nutrition Res Rev 1999; 12: 231-253.
- Tomaszewska E, Dobrowolski P, Puzio I. Postnatal administration of 2-oxoglutaric acid improves the intestinal barrier affected by the prenatal action of dexamethasone in pigs. Nutrition 2012; 28: 190-196.
- Sliwa E, Dobrowolski P, Piersiak T. Bone development of suckling piglets after prenatal, neonatal or perinatal treatment with dexamethasone. J Anim Physiol Anim Nutr (Berl) 2010; 94: 293-306.
- Tomaszewska E, Dobrowolski P, Wydrych J. Postnatal administration of 2-oxoglutaric acid improves articular and growth plate cartilages and bone tissue morphology in pigs prenatally tretaed with dexamethasone. J Physiol Pharmacol 2012; 63: 547-554.
- Vahakangas K. Chemical exposure as etiology in developmental origin of adult onset human cancer. Front Pharmacol 2011; 2: 62.
- Yaylayan VA, Wnorowski A, Perez Locas C. Why asparagine needs carbohydrates to generate acrylamide. J Agric Food Chem 2003; 51: 1753-1757.
- Tyla RW, Friedmanb MA, Losco PE, et al. Rat two-generation reproduction and dominant lethal study of acrylamide in drinking water. Reprod Toxicol 2000; 14: 385-401.
- Hulas-Stasiak M, Dobrowolski P, Tomaszewska E, Kostro K. Maternal acrylamide treatment reduces ovarian follicle number in newborn guinea pig offspring. Reprod Toxicol 2013; 42: 125-131.
- Gokmen V, Palazoglu TK. Measurement of evaporated acrylamide during frying of potatoes: effect of frying conditions and surface area-to-volume ratio. J Food Eng 2009; 93: 172-176.
- Schettgen T, Rossbach B, Kutting B, Letzel S, Drexler HJ. Determination of haemoglobin adducts of acryloamide and glycidamide in smoking and non-smoking persons of the general population. Int J Hyg Environ Health 2004; 207: 531-539.
- Dobrowolski P, Huet P, Karlsson P, et al. Potato fibre protects the small intestine wall against the toxic influence of acrylamide. Nutrition 2012; 28: 428-435.
- Sorgel F, Weissenbacher R, Kinzig-Schippers M, et al. Acrylamide: increased concentrations in homemade food and first evidence of its variable absorption from food, variable metabolism and placental and breast milk transfer in humans. Chemotherapy 2002; 48: 267-274.
- FAO/WHO Consultation on the Health Implications of Acrylamide in Food. Geneva, 25-27 June 2002.
- Merchant HA, McConnell EL, Liu F, et al. Assessment of gastrointestinal pH, fluid and lymphoid tissue in the guinea pig, rabbit and pig, and implications for their use in drug development. Eur J Pharm Sci 2011; 42: 3-10.
- El-Bakry AM, Abdul-Hamid M, Allam A. Prenatal and perinatal exposure of acrylamide disrupts the development of spinal cord in rats. World J Neurosci 2013; 3: 17-31.
- Allam AA, El-Ghareeb AW, Abdul-Hamid M, Bakery AE, Gad M, Sabri M. Effect of prenatal and perinatal acrylamide on the biochemical and morphological changes in liver of developing albino rat. Arch Toxicol 2010; 84: 129-141.
- Allam A, El-Ghareeb A, Abdul-Hamid M, Baikry A, Sabri M. Prenatal and perinatal acrylamide disrupt s the development of cerebellum in rat: biochemical and morphological studies. Toxicol Ind Health 2011; 27: 291-306.
- ILAR/Subcommittee on Laboratory Animal Nutrition Committee on Animal Nutrition, Board on Agriculture, National Research Council. Nutrient Requirements of Laboratory Animals. Washington, DC, National Academies Press, 1995.
- Tyl RW, Friedman MA. Effects of acrylamide on rodent reproductive performance. Reprod Toxicol 2003; 17: 1-13.
- Suvarna SK, Layton C, Bancroft JD. Bancroft's Theory and Practice of Histological Techniques. Churchill Livingstone Elsevier 2013.
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 1979; 11: 447-455.
- Greca FH, Noronha L, Marcolini FRN, Verona A, Pereira IA, Bier RS. Small intestinal submucosa as a graft to increase rectum diameter. J Surgical Res 2013; 183: 503-508.
- Abu Eid R, Landini G. Quantification of the global and local complexity of the epithelial-connective tissue interface of normal, dysplastic, and neoplastic oral mucosae using digital imaging. Pathol Res Pract 2003; 199: 475-482.
- Slupecka M, Wolinski J, Pierzynowski SG. The effects of enteral ghrelin administration on the remodeling of the small intestinal mucosa in neonatal piglets. Regul Pept 2012; 174: 38-45.
- Kisielinski K, Willis S, Prescher A, Klosterhalfen B, Schumpelick V. A simple new method to calculate small intestine absorptive surface in the rat. Clin Exp Med 2002; 2: 131-135.
- Lizard G, Fournel S, Genestier L, et al. Kinetics of plasma membrane and mitochondrial alterations in cells undergoing apoptosis. Cytometry 1995; 21: 275-283.
- Majno G, Joris I. Apoptosis, oncosis, and necrosis an overview of cell death. Am J Pathol 1995; 146: 3-15.
- Lopachin RM, Gavin T. Acrylamide-induced nerve terminal damage: relevance to neurotoxic and neurodegenerative mechanisms. J Agr Food Chem 2008; 56: 5994-6003.
- Sirota V, Hommet F, Tard A, Leblanc JC. Dietary acrylamide exposure of the French population: results of the second French Total Diet Study. Food Chem Toxicol 2012; 50: 889-894.
- Tomaszewska E, Dobrowolski P. Maternal glucocorticoid treatment as a model for examining foetal gender-specific effects on the development that influences the bone metabolism of neonatal piglets. Bull Vet Inst Pulawy 2012; 56: 247-253.
- Tomaszewska E, Dobrowolski P, Siwicki AK. Maternal treatment with dexamethasone at minimal therapeutic doses inhibits neonatal bone development in a gender-dependent manner. Livestock Sci 2012; 146: 175-182.
- Mickiewicz M, Zabielski R, Grenier B, et al. Structural and functional development of small intestine in intrauterine growth retarded porcine offspring born to gilts fed diets with differing protein ratios throughout pregnancy. J Physiol Pharmacol 2012; 63: 225-239.
- Moonen RM, Kessels CG, Zimmermann LJ, Villamor E. Mesenteric artery reactivity and small intestine morphology in a chicken model of hypoxia-induced fetal growth restriction. J Physiol Pharmacol 2012; 63: 601-612.
- Friedman M. Chemistry, biochemistry, and safety of acrylamide. A review. J Agric Food Chem 2003; 51: 4504-4526.
- Armstrong DJ. Food chemistry and U.S. food regulations. J Agric Food Chem 2009; 57: 8180-8186.
- Mucci LA, Wilson KM: Acrylamide intake through diet and human cancer risk. J Agric Food Chem 2008; 56: 6013-6019.
- Granby K, Fagt S. Analysis of acrylamide in coffee and dietary exposure to acrylamide from coffee. Analyt Chim Acta 2004; 520: 177-182.
- Mojska H, Gielecinska I, Szponar L, Oltarzewski M. Estimation of the dietary acrylamide exposure of the Polish population. Food Chem Toxicol 2010; 48: 2090-2096.
- Fennell TR, Sumner SC, Snyder RW, et al. Metabolism and hemoglobin adduct formation of acrylamide in humans. Toxicol Sci 2005; 85: 447-459.
- Kelly D, King TP. Luminal bacteria: regulation of gut function and immunity. In: Manipulation of the Gut Environment in Pig. A. Piva, K.E. Bach Kudsen, J.E. Lindberg (eds.). Nottingham, Nottingham University Press, 2001; pp. 113-131.
- Hang CH, Shi JX, Li JS, Wu W, Yin HX. Alterations of intestinal mucosa structure and barrier function following traumatic brain injury in rats. World J Gastroenterol 2003; 9: 2776-2781.
- Zodl B, Schmid D, Wassler G, et al. Intestinal transport and metabolism of acrylamide. Toxicology 2007; 232: 99-108.
- El-Sayyad HI, Abou-Egla MH, El-Sayyad FI, et al. Effects of fried potato chip supplementation on mouse pregnancy. Nutrition 2011; 27: 343-350.
- Gamboa Da Costa G, Churchwell MI, Hamilton LP, et al. DNA adducts formation from acrylamide <i>via</i> conversion to glycidamide in adult and neonatal mice. Chem Res Toxicol 2003; 16: 1328-1337.
- Doerge DR, Young JF, McDaniel LP, Twaddle NC, Churchwell MI. Toxicokinetics of acrylamide and glycidamide in Fischer 344 rats. Toxicol Appl Pharmacol 2005; 208: 199-209.
- Morita K, Furuse M, Fujimoto K., Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA 1999; 96: 511-516.
- Brantsaeter AL, Haugen M, de Mul A, et al. Exploration of different methods to assess dietary acrylamide exposure in pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa). Food Chem Toxicol 2008; 46: 2808-2814.
A c c e p t e d : December 30, 2013