OVERVIEW OF THE ROLE OF VITAMINS AND MINERALS
ON THE KYNURENINE PATHWAY IN HEALTH AND DISEASE
INTRODUCTION
The kynurenine pathway (KP) of L-tryptophan metabolism is increasingly recognised as a critical source of neuroactive metabolites with relevance for health and disease. There is mounting evidence that L-tryptophan metabolism depends on the adequate availability of both vitamins (1-10) and minerals (11-19) which work as cofactors and coenzymes in metabolic reactions. L-tryptophan is an "essential" amino acid for mammals, as they cannot make it themselves. Therefore, they must obtain it in the form of food. This aromatic substance can be utilized not only for protein synthesis, but also for the biosynthesis of nicotinamide adenine dinucleotide (NAD+) through the KP, as well as the biosynthesis of serotonin and melatonin through the serotonin pathway (20).
L-tryptophan has been proven to modulate gene expression and nutrient metabolism with an impact on whole-body homeostasis. It has been demonstrated that a temporary L-tryptophan deficiency can cause memory failure in healthy humans; as well as dermatitis, depression, and anxiety (20, 21). A low dietary tryptophan intake during rat development produced lower rates of serotonin synthesis in the brain, affecting GABAergic cortical interneurons and contributing to an increased susceptibility to convulsions (22).
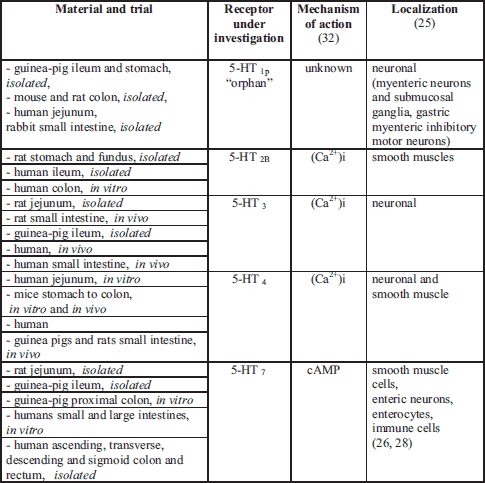
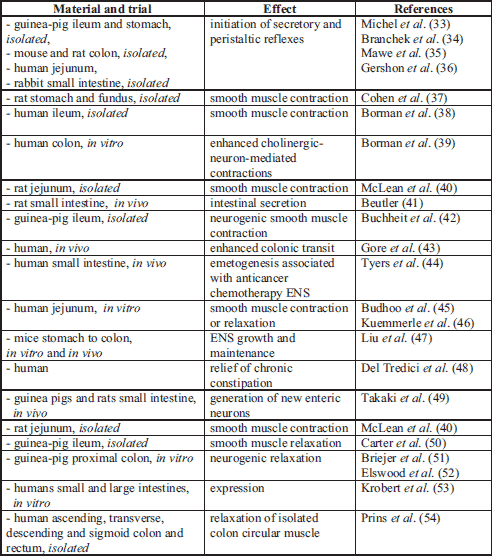
ENS, enteric nervous system.
In a preclinical study of pregnant rats, an excess of L-tryptophan in the diet caused hyperserotonaemia in the rat foetus, which had an adverse effect on the development of serotonergic neurons, thus affecting the growth of new-born rats (23). On the other hand, Strasser et al. (24) point out that L-tryptophan supplementation while dieting could be highly useful in preventing neuropsychiatric symptoms or in treating uncontrolled weight gain in mammals.
Serotonin is an important neurotransmitter in the enteric nervous system (ENS) (25-28), in the central nervous system (CNS) (29), as well as in the autonomic nervous system (ANS). In the ENS, serotonin regulates secretion, motility and sensation (Table 2), while in the CNS it modulates mood, cognition and sleep (20, 29). Serotonin also regulates the secretion of pituitary growth hormone, which in turn stimulates the liver to produce insulin-like growth factor-I, which is necessary for the development and growth of rat pups as well as other mammals (23). Neuropsychiatric problems have been attributed to too much or too little serotonin (27, 29, 30). Recent studies have proven, that changes in serotonin concentration play a crucial role in human gastrointestinal (GI) physiology, being a therapeutic target in irritable bowel syndrome (IBS) etiology (26, 27, 31).
The other product of L-tryptophan metabolism is melatonin. It has been shown in several studies to have free radical scavenging activity (55), antioxidant properties (56), anti-apoptotic activity (57), and enhancement of mitochondrial function (58).
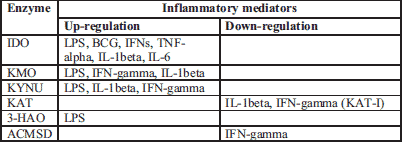
In recent years, attention has increasingly focused on the medical significance of the enzymatic oxidation of L-tryptophan which is dependent on some B group vitamins and minerals to form compounds collectively known as "kynurenines". Segregated into at least two distinct branches, often termed as the "neurotoxic" and "neuroprotective" arms of the KP, they are regulated by the two key enzymes, flavin dependant (FAD) kynurenine 3-monooxygenase (KMO) and pyridoxal-5'-phosphate (PLP, vitamin B6) dependent kynurenine aminotransferase (KAT), respectively (59). These enzymes are mainly located in the brain (being segregated into specific cell types) (60), in the liver and kidney (59) as well as throughout the body. Several of these enzymes are under the tight control of inflammatory mediators (59, 61) (Table 3).
The KP produces several neuroactive metabolites. The neurotoxic ones are: amino acid 3-hydroxy-L-kynurenine (3-OH-L-KYN), 3-hydroxy-anthranilic acid (3-OH-AA), and quinolinic acid (QUIN). The neuroprotective metabolite is kynurenic acid (KYNA) (60, 62). Moreover, the KP is the major route of L-tryptophan metabolism (95% of all ingested L-tryptophan) that leads to the formation of the active form of niacin (NAD+) in the main chain (63). Meanwhile, the side-chains of this pathway generate KYNA and xanthurenic acid (XA) (64) (Fig. 1).
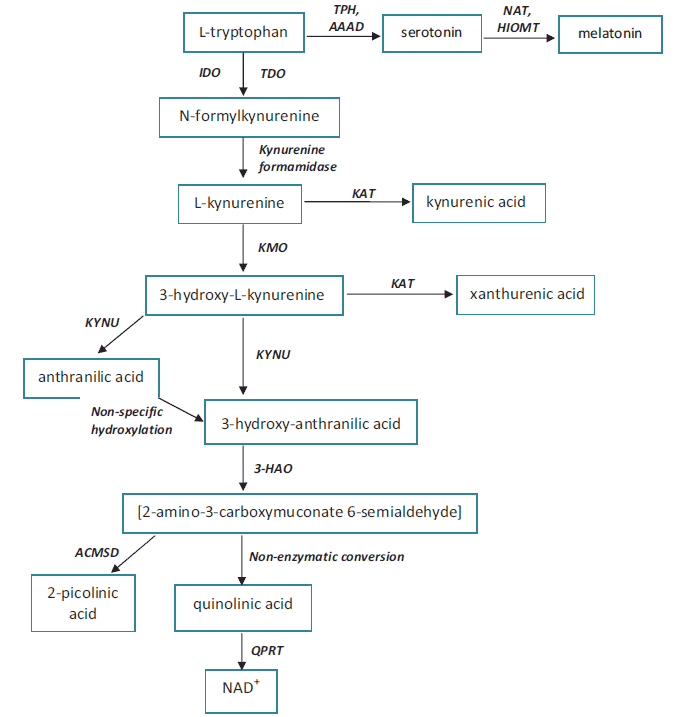
QUIN is considered to be an endogenous agonist of the N-methyl-D-aspartate (NMDA) receptor with strong excitotoxic properties (65). By contrast, KYNA possesses a neuroprotective action (in the CNS), and is an NMDA receptor antagonist as well as a non-competitive antagonist of the nicotinic receptor a-A7 subtype (66). What's more, QUIN, 3-OH-L-KYN and 3-OH-AA are responsible for the production of the extremely reactive free radical species, O2●- and H2O2 (62).
Some health related states may influence the proper functioning of the KP (Table 4). Excessive L-kynurenine (L-KYN) production and an accompanying deficiency of the substrate L-tryptophan, may be the cause of a number of pathological conditions. According to Sasaki et al. (104), insulin-dependent diabetes mellitus in rats may alter the activity of enzymes engaged in L-tryptophan metabolism, which may lead to changes in the production of different kynurenines. Matsuda et al. (105) recently found in rats that another KP enzyme - α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase (ACMSD) - is also associated with proper cholesterol metabolism. This enzyme is dependent on proper concentrations of Fe2+, Co2+ and Zn2+ (13).

Abbreviations: 3-OH-AA, 3-hydroxy-antranilic acid; 3-OH-L-KYN, 3-hydroxy-L-kynurenine; 5-HT, serotonin; AA, anthranilic acid; IBS, irritable bowel syndrome; KYNA, kynurenic acid; L-KYN, L-kynurenine; L-TRP, L-tryptophan; PA, picolinic acid; QUIN, quinolinic acid; XA, xanthurenic acid.
L-tryptophan utilization as a fuel for energy production is dependent upon the adequate availability of both vitamins and minerals such as: pyridoxal 5'-phosphate (PLP, B6), riboflavin (B2), niacin (B3), thiamine (B1), pantothenic acid, lipoate, ubiquinone, Mg2+, Fe2+, Zn2+ and Co2+ (106). Consequently, a lack of certain vitamins and minerals may lead to the dysfunction of enzymes engaged in L-tryptophan metabolism and many health-related disorders (107) (Table 4).
ENZYMES ENGAGED IN TRYPTOPHAN METABOLISM
ALONG THE KYNURENINE PATHWAY
L-kynurenine to L-tryptophan ratio as a tryptophan and indoleamine 2,3-dioxygenase indicator
The first and rate-limiting step of the KP is the oxidative cleavage of the 2,3-double bond of the indole ring of L-tryptophan to form an unstable product (N-formyl-L-kynurenine, NFK) which is further converted to L-KYN. This reaction is catalysed by two iron dependent dioxygenases (122, 123). They are tryptophan 2,3-dioxygenase (TDO), which is constitutively expressed in liver; and the inflammatory cytokine-induced indoleamine 2,3-dioxygenase-1 (IDO-1) which is located in the brain and in the intestine (124). The enzyme catalyses the oxidation of L-tryptophan to L-KYN in a reaction that produces peroxides and gives rise to highly reactive and potentially harmful oxygen and hydroxyl radicals (62). The heme prosthetic group of TDO-heme-Fe3+ must be reduced to the heme-Fe2+ form prior to mediating L-tryptophan oxidation (122, 125). Like other hemeproteins, IDO binds O2 to the deoxy protein where it is involved in the oxidation of substrates (Table 4). It was proven that IDO activity is dependent on the proper concentration of Fe2+ and many inflammatory stimuli (Table 2).
The plasma (serum) ratio of L-KYN to L-tryptophan (K-TR ratio) is a generally accepted clinical marker of IDO activity (126). It is highly correlated to neopterin levels, which is a macrophage-derived metabolite that increases after interferon-gamma stimulation, and is a good reflection of inflammatory status (90). Considering that both IDO and TDO regulate the rate of L-tryptophan conversion into L-KYN, plasma concentrations of L-tryptophan and L-KYN might also be affected by the activity of TDO (21). However, the K-TR ratio most accurately reflects IDO activity in conditions associated with inflammation (91).
More recently, it has been found that mammals possess a third dioxygenase enzyme, in addition to TDO and IDO-1. IDO-2's active site contains a heme moiety for L-tryptophan binding and catabolism (127). This enzyme is capable of catalysing the oxidative cleavage of L-tryptophan, and is subsequently named indoleamine-2,3-dioxygenase type 2 (IDO-2) with a role in immunity (128). The discovery of a third initiating protein of the KP has attracted considerable research interest due to the established roles of IDO-2 in L-tryptophan metabolism (129) and TDO/IDO-1 dysfunction in numerous pathological conditions, including malaria (123, 130) and cancer (131).
An overexpression of IDO activity is connected with human cancer development (109, 110), IFN-α-induced depression (132) and IBS development (111, 112). Cytokines up-regulate IDO expression (132), causing a decrease in extracellular serotonin (Table 3). This can induce an increase in sleep, reduce locomotor activity, and decrease social exploration with co-existing anhedonia in animal models. These symptoms resemble the vegetative symptoms of depression in humans (133). Furthermore, dysregulation of L-tryptophan metabolism together with vitamin B6 insufficiency influences serotonin and melatonin formation, with an impact on motility and perception in the ENS (124).
In IBS patients, plasma kynurenine levels are significantly elevated as is the K-TR ratio, an index of IDO activity, in comparison to control levels (134).
The inhibition of IDO activity with L-tryptophan loading, exerts protective effects by suppressing the formation of neurotoxic QUIN, 3-OH-L-KYN, 3-OH-AA, and nitric oxide synthase (135), and increases melatonin formation. Therefore this could be a new therapeutic approach in the treatment of many CNS and ENS disorders. Moreover, down-regulation of IDO, leads to a reduction of proinflammatory cytokine activity (59).
Kynurenine-3-monooxygenase (kynurenine 3-hydroxylase)
KMO catalyses the specific hydroxylation of L-KYN at the third position of its phenol ring to generate 3-OH-L-KYN, an endogenous oxidative stress generator, which causes neuronal cell death with apoptotic features and regional selectivity (136). The mechanism of toxicity to the cells is through the generation of reactive oxygen species in the respiratory chain (137).
KMO is up-regulated by FAD coenzyme (a form of vitamin B2) and its monomeric protein is non-covalently and very tightly bound to FAD with the ability to slow chemical dissociation (1). NADPH, NADH, FAD and inflammatory stimuli up-regulate enzyme activity, whereas AA, XA and most likely vitamin B6 are the negative regulators of the enzyme activity (62) (Table 3 and Table 5). In vitamin B6 deficiency, KMO activity is decreased, which may further lead to the development of other neurological disorders (10, 108).
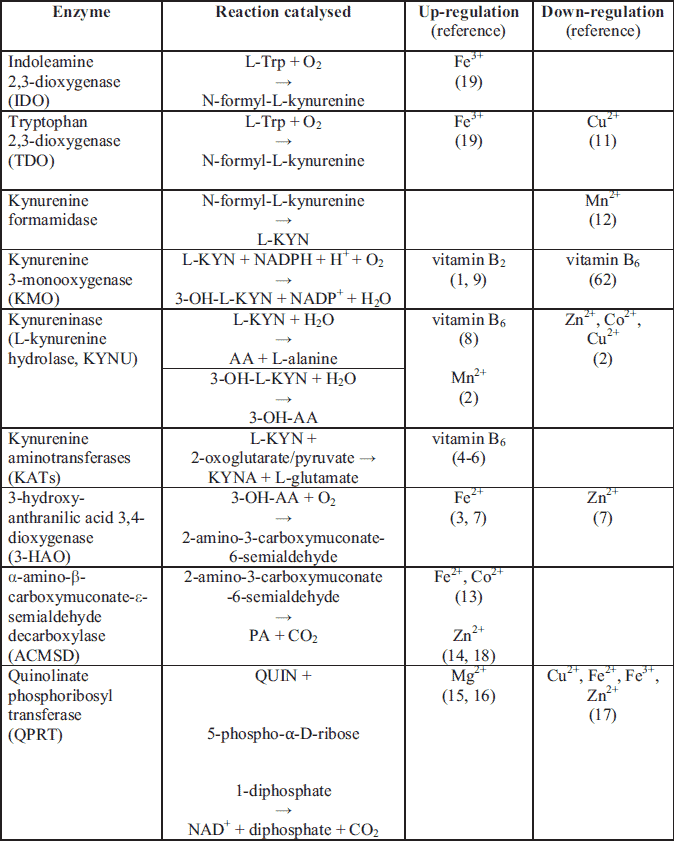
Physiologically, high KMO levels are found in the liver and kidney, as well as within macrophage and microglial cells, whereas low KMO levels are distributed throughout all regions of the brain (136). More thorough studies further indicate that the enzyme is localized upon the outer mitochondrial membrane (138-140).
Recently, KMO has been proven to be involved in the pathogenesis of Huntington's disease (113), post-traumatic sepsis (138), Alzheimer's disease (141), and is considered to be an important pharmaceutical target for the development of drugs against neurodegenerative diseases (142, 143). Increased concentrations of 3-OH-L-KYN have been found in the disorders described above. Thus, KMO inhibition can be a potential therapeutic target for the treatment of neurodegenerative diseases (1, 114).
Kynureninase (L-kynurenine hydrolase)
KYNU is a vitamin B6 dependent enzyme which lies on the main pathway towards acetyl-CoA or NAD+ synthesis. Both L-KYN and 3-OH-L-KYN can be oxidized to AA or 3-OH-AA, respectively (144). L-KYN to AA and 3-OH-L-KYN to 3-OH-AA ratios are considered to be the KYNU indicators (Table 4). The role of 3-OH-L-KYN in health and disease is controversial as there have been reports regarding its pro-oxidant and antioxidant properties (62).
In parallel with KYNU, another vitamin B6 dependent enzyme, KAT, utilizes the same reactants but obtains XA and KYNA as products instead (116).
In vitamin B6 deficiency states and in pregnancy, L-KYN and 3-OH-L-KYN predominate, suggesting a block in the pathway at the level of KYNU (76, 77). What is more, the KAT metabolites also exist, suggesting that although both enzymes require vitamin B6, KYNU has been shown to be more sensitive to vitamin B6 depletion than KAT (Table 7). Thus, depletion in vitamin B6 concentration shifts the pathway towards the formation of KYNA and XA (84). Pregnancy exerts a more pronounced effect on KYNU activity than vitamin B6 restrictions, and the effects of pregnancy and diet are additive (76, 77). In another study of vitamin B6 deficiency, KYNU activity in the kidneys was significantly reduced, while KAT was increased (145). Moderate deficiency of vitamin B6 causes an increase in the concentration of 3-OH-L-KYN whereas KYNA and AA concentration are decreased (146). According to Takeuchi et al. (145), the quantity of KYNA was highly increased. More serious deficiencies lead to an increase in the concentration of L-KYN and XA in blood and urine (146-149). The increase in the concentration of 3-OH-L-KYN is an important indicator of abnormalities occurring in the metabolism of vitamin B6. Vitamin B6 deficient animals excrete more XA and 3-OH-L-KYN, and less of the niacin metabolites (N-1-methyl nicotinamide and methyl-2-pyridone-4-carboxamide), when compared to the control group after L-tryptophan loading (84, 150). The 3-OH-L-KYN : 3-OH-AA ratio, which is a substrate-product ratio of KYNU, was proposed as the most sensitive and specific indicator of increased vitamin B6 dependency (151).
A recent study shows that minerals can also exert an influence on KYNU activity. According to studies conducted by El-Sewedy et al. (2), Mn2+ can activate the enzymes, whereas Zn2+, Co2+ and Cu2+ inhibit enzymatic activity. This inhibition is attributed to the blocking and inactivation of the sulfhydryl groups of the enzyme. The decreasing order by which these metal ions inhibit KYNU is Cu2+ > Co2+ > Zn2+.
Additionally, KYNU is inactivated by incubation with a reaction product, L-alanine, and its function is restored by the addition of vitamin B6 (153). The inhibition of KYNU is associated with sedative and anticonvulsant actions (154) (Table 4). On the other hand, interferon-gamma stimulation significantly potentiates the expression of KYNU in cultured human glioma cells (61) (Table 3).
Kynurenine aminotransferases
Kynurenine aminotransferases, as implied by their names, are the primary enzymes involved in the irreversible transamination of KYNA from L-KYN in human brain tissue, and are referred to as KATs. KATs are homodimeric pyridoxal proteins that mediate this catalytic conversion (4). Although four isoforms (KAT-I to -IV) of this enzyme have been hitherto identified, KAT-II is the enzymatic isoform that mainly accounts for the synthesis of cerebral KYNA (155). KAT is also involved in the biosynthesis of glutamic (156, 157) and aspartic acid (4), which function as neurotransmitters for the NMDA receptors in mammals (158) (Table 5). Moreover, KAT is responsible for the transamination of 3-OH-L-KYN to XA in the competing, major branch of the metabolic cascade. However, this function of KAT has been rarely highlighted in the published literature as of yet (159). KATs are not sensitive to changes in minerals; however, there are some reports suggesting its sensitivity to vitamin B6 fluctuations (Table 7). The data as of present have shown little effect of dietary vitamin B6 on KATs' activity (76, 77).
KAT-I is potently inhibited by glutamine, whereas KAT-II is not sensitive to glutamine. KAT-I that is located in the brain is inhibited by competing substrates, like L-tryptophan, L-phenylalanine and L-glutamine, which are present in the human body under physiological conditions (160). On the other hand, KAT-I that is located in the heart is not inhibited by the afore-mentioned compounds (161).
It was reported that selective inhibitors of KAT-II restore nicotine-evoked glutamatergic activity in the cortex, being a possible treatment target for such cognitive impairments (162), as KAT-II is responsible for most of the KYNA synthesis in the brain. Notably, compounds targeting KAT-II selectively may provide clinical benefits in a host of psychiatric and neurological disorders by normalizing the function of brain KYNA and its receptors (116, 163).
The product of KAT activity - KYNA is known to be a highly neuroactive metabolite whose impairment is associated with a number of severe brain disorders such as Alzheimer's disease (164), schizophrenia (165) ischemic stroke (166), and with type 2 diabetes (88) (Table 6). High levels of KYNA have been identified in human urine during marked vitamin B6 deficiency. Kocki et al. (167) has proven that the amino acid L-cysteine can down-regulate KYNA formation in the brain. A study among acute ischemic stroke patients revealed decreased activity of KAT, KYNA, L-tryptophan, and apolipoprotein A1 (166). Pregnancy results in an overall reduction of enzyme activity; however, the degree of reduction is minimal, and pregnancy does not enhance the effect of vitamin B6 restriction.

The role of a second product, XA, and the influence of vitamins and minerals on its production is not well known. XA accumulates in pre-synaptic terminals and is intensively produced and excreted in urine after the ingestion of L-tryptophan during vitamin B6 deficiency (145). Copeland (168) suggests that XA may be the first potential endogenous allosteric agonist (termed "endocoid") for the metabotropic glutamate receptors. Additionally, XA may participate in apoptotic events in vascular smooth muscle and retinal pigment epithelium cells with modifications in mitochondrial Ca2+ transport and oxygen consumption (169).
3-hydroxy-anthranilic acid 3,4-dioxygenase
3-HAO is an iron dependent enzyme requiring O2, Fe2+ and sulfhydryl groups for its activation (3, 170, 171). This enzyme is mainly localized to hepatic tissue in the mitochondrial membrane, but is also expressed in the brain, mainly in astrocytes (172), in the liver, and in the inflammatory cells (65). There are no structural differences between the enzyme from the brain and the liver, except that higher amounts of 3-HAO have been observed in the liver (173).
3-HAO activity may normally be restrained by certain factors such as the availability of Fe2+ ions and O2. The addition of Fe2+ stimulates the enzyme's activity 4- to 6-fold in striatal homogenates of mouse, rat, and human; and can be inhibited by ferritin (176). Iron released during neuronal damage stimulates 3-HAO and inhibits QPRT, thus significantly elevating the production of QUIN, which thereby causes more damage to the CNS. On the other hand, Zn2+ was described as having a negative impact on enzyme activity (7). Moreover iron ions are responsible for 3-OH-L-KYN induced neuronal cell death either by catalysing the oxidation of 3-OH-L-KYN or in promoting the production of highly reactive hydroxyl radicals (136, 152).
The role of 3-HAO in proper CNS functioning is still unclear and controversial. The activity of 3-HAO is significantly reduced in schizophrenia (73, 74), Huntington's disease (85), chronic brain injury (118), stroke (119), depression (120), and osteoporosis (121) (Table 6). In pathology, the normal ratio between 3-OH-AA and AA is also changed, with lower levels of 3-OH-AA and higher levels of AA than normal (175). There are also reports suggesting that 3-HAO plays anti-inflammatory and neuroprotective roles during inflammation, and thus may have implications for future therapeutic approaches for neuroinflammatory disorders (174). Thus, it is justifiable that reducing levels of toxic QUIN by administering such inhibitors of the enzymes of the KP, particularly 3-HAO, may be a reasonable approach for the treatment of such cognitive impairments (7).
2-amino 3-carboxymuconate 6-semialdehyde decarboxylase
ACMSD diverts 2-amino-3-carboxymuconate semialdehyde from QUIN production, towards picolinic acid (PA) formation, and thus is responsible for the proper homeostasis between PA, QUIN and NAD+ (Fig. 1). ACMSD is a Zn-dependent amidohydrolase (14) and is activated slightly by Fe2+ and Co2+ (13) (Table 7). On the other hand, QUIN, PA and KYNA inhibit ACMSD activity (13). Furthermore, interferon-gamma significantly down-regulates the expression of both ACMSD and KAT-I in cultured human glioma cells (61); however, it significantly potentiates the expression of IDO-1, IDO-2, KYNU, and KMO (Table 3). ACMSD is present in kidney and liver, with its highest expression occurring in kidney (13). As the relative concentrations of PA and QUIN must be tightly controlled, modulation of ACMSD may be another key to the treatment of neurological disorders (Table 4). Therefore, ACMSD is an attractive therapeutic target for treating disorders associated with increased levels of neurotoxic QUIN, and factors which directly influence enzyme activity may be an interesting approach to modulate L-tryptophan metabolism.
An increased concentration of bivalent metals such as Fe2+, Co2+ and Zn2+ may increase the concentration of PA, thereby decreasing QUIN formation.
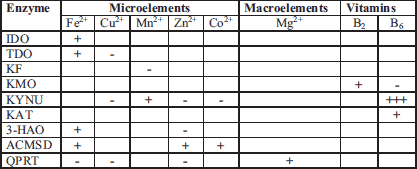
"+" up-regulation; "-" down-regulation
Interestingly, PA may inhibit QPRT activity through Zn2+ chelation, thus reducing QUIN induced neurotoxicity (177).
Quinolinate phosphoribosyl transferase
QPRT has been identified in the CNS of both rats and humans (178, 179). The role of QPRT is to catabolize QUIN to NAD+ with carbon dioxide as a by-product. QUIN is often implicated in the pathogenesis of a variety of human neurological diseases, as it is an agonist of the NMDA receptor (158), and has a high in vivo potency as an excitotoxin (177). NAD+ is an important enzymatic cofactor that is vital to ongoing cell viability and maintenance of mechanisms such as the DNA repair protein poly(ADP-ribose) polymerases (180). Under physiological conditions, Mg2+ is required for QPRT activation with further evidence that a cysteine residue at the active site is required for reaction catalysis (178) (Table 7). The proper functioning of QPRT, which is dependent on Mg2+ intake, prevents the accumulation of toxic QUIN (Table 5). Mg2+ insufficiency may lead to the inhibition of enzyme, thus promoting accumulation of neurotoxic reactants. Grimaldi et al. (103) point out that Mg2+ deficiency may be the central precipitating event resulting in the symptomatology of Tourette's syndrome and several reported comorbid conditions. As of now, it is not known if Mg2+ supplementation influences enzymatic activity during the development of QUIN-induced toxicity of neurological processes, as free radical generation and oxidative stress are involved in this process.
On the other side of the coin, Cu2+ Fe2+, Fe3+, Zn2+ deficiencies are considered to up-regulate QPRT activity, and thus may inhibit QUIN accumulation (17, 62). Moreover, the complex formation between QUIN and excess Fe2+ can produce oxidative cell damage through lipid peroxidation and can inhibit the auto-oxidation of the Fe2+ complex which is responsible for in vitro DNA chain breakage (181). Thus, an excess of Fe2+ plays an etiologically significant role in neurodegenerative disorders (176). Pathologic accumulation of QUIN has been found in neurodegenerative disorders including Alzheimer's (99) and Huntington's disease (100) and has also been connected with the aging process (101, 102) (Table 4). Moreover, Sahm et al. (172) identifies QPRT as a potential therapeutic target in malignant gliomas. Sasaki et al. (104) suggest that the immune and neuronal systems of insulin dependent diabetes mellitus would be influenced by the increased amounts of QUIN and L-KYN, but not by those of PA and NAD+ as diabetes mellitus does not augment the latter species. Treatment with metal chelates such as PA demonstrate significant neuroprotection against QUIN-formation, responsible for excitotoxic damage to the brain, towards NAD+ formation (177).
CONCLUSIONS
Under pathological conditions, the concentrations of L-tryptophan, vasoactive L-kynurenine, neuroactive KYNA, QUIN, 3-OH-L-KYN, 3-OH-AA, and enzymes responsible for their formation (IDO, KMO, KAT, KYNU, 3-HAO) are significantly changed in blood, in urine and in the brain (182) (Table 4). Several of these enzymes are under the tight control of inflammatory mediators (59, 61). Also, the concentration of the essential amino acid L-tryptophan was reported to be decreased by the pro-inflammatory cytokines (59, 61). In septic shock patients, the concentration of L-tryptophan is decreased, as opposed to L-KYN which is intensively produced (75). Studies performed by Celinski et al. (183) demonstrate the protective effect of melatonin and L-tryptophan by reducing concentrations of pro-inflammatory Il-1, Il-6 and TNF-α. Also the concentrations of B group vitamins (76, 77, 86, 89, 107, 126, 146, 150, 151) and minerals are significantly altered (Table 5).
The evidence presented in this paper suggests that the KP is highly sensitive to changes in the concentration of B group vitamins (B2, B6) as well as micro- (Fe3+, Mn2+, Zn2+, Cu2+, Co2+) and macro-elements (Mg2+). Both vitamins and minerals can work as coenzymes and cofactors in de novo synthesis of another B-vitamin niacin. Another pathway of L-tryptophan metabolism, the serotonin pathway, is also dependent on proper diet and vitamin B6 status (184-186), which suggests that any alterations to L-tryptophan metabolism might also influence the production and activity of both serotonin and melatonin (187).
Minerals and B group vitamins at physiological concentrations can either directly up-regulate and/or down-regulate the activity of enzymes engaged in L-tryptophan metabolism (Table 7). Vitamin B6 was found to be the most crucial vitamin engaged in L-tryptophan metabolism, since it is involved in the proper functioning of the serotonin pathway enzyme tryptophan hydroxylase, and the kynurenine pathway enzymes KYNU and KAT.
However, surplus administration of B-group vitamins did not elicit beneficial effects on L-tryptophan metabolism, since deficiencies rather than surpluses seem to influence the KP (107). The administration of minerals in a normally balanced diet is not known to influence the KP.
Nutrient deficiencies plus coexisting physiological processes may have hyper-additive effects on biological systems, therefore causing increased harmful effects (76, 77). In other words, in alcoholics, people experiencing decreased food intake, and during high physical activity when the needs are increased (188), the concentration of L-tryptophan in the diet is not sufficient. Any diseases to the GI-tract influence both proper food absorption and GI synthesis of many organic compounds such as melatonin, serotonin or kynurenines in the gut (185). Thus, it is crucial to maintain proper functioning of the GI-tract, which will also accelerate the healing processes (189).
Pharmacological down-regulation of the L-tryptophan - L-KYN - NAD+ pathway together with a tryptophan-rich diet and maintenance of adequate B vitamins and minerals is important for patients susceptible to depression, diabetes, post-traumatic stress disorder, chronic pain, cancer, and drug addiction (186). It is likely that this regulation can also influence the progression of epilepsy, Parkinson's disease, Alzheimer's disease or schizophrenia (141). Other conditions associated with inflammation and stress-induced excess of L-KYN production that coincide with vitamin B6 deficiency are obesity, cardiovascular diseases, aging, pre-and postmenstrual phase in certain females, pregnancy, and hepatitis C virus infection (91).
It is therefore reasonable to assume that nutrients that affect the enzymes involved in the formation or metabolism of the "kynurenines" are useful tools that may improve our understanding of the role of those enzymes in physiology and pathology. However, much more diversified research is still needed to fully understand the complex interaction between enzymes and B group vitamins and minerals, how they react with each other, how they react within the nervous systems, as well as how they react on the periphery.
Abbreviations: 3-HAO, 3-hydroxy-anthranilic acid dioxygenase; 3-OH-AA, 3-hydroxy-antranilic acid; 3-OH-L-KYN, 3-hydroxy-L-kynurenine; 5-HT, serotonin; AA, anthranilic acid; CNS, central nervous system; ENS, enteric nervous system; FAD, flavin adenine dinucleotide; IBS, irritable bowel syndrome; IDO, indoleamine 2,3-dioxygenase; KAT, kynurenine aminotransferase; KF, kynurenine formamidase; KMO, kynurenine 3-monooxygenase; KP, kynurenine pathway; KYNA, kynurenic acid; KYNU, kynureninase or L-kynurenine hydrolase; L-KYN, L-kynurenine; L-TRP, L-tryptophan; NAD+, nicotinamide adenine dinucleotide; NMDA, N-methyl-D-aspartate; PA, picolinic acid; QPRT, quinolinate phosphoribosyl transferase; QUIN, quinolinic acid; TDO, tryptophan 2,3-dioxygenase; XA, xanthurenic acid.
Conflict of interests: None declared.
REFERENCES
- Amaral M, Levy C, Heyes DJ, et al. Structural basis of kynurenine 3-monooxygenase inhibition. Nature 2013; 496: 382-385.
- El-Sewedy SM, Abdel-Tawab GA, El-Zoghby SM, Zeitoun R, Mostafa MH. Studies with tryptophan metabolites in vitro. Effect of zinc, manganese, copper and cobalt ions on kynurenine hydrolase and kynurenine aminotransferase in normal mouse liver. Biochem Pharmacol 1974; 23: 2557-2565.
- Zhang Y, Colabroy KL, Begley TP, Ealick SE. Structural studies on 3-hydroxyanthranilate-3,4-dioxygenase: the catalytic mechanism of a complex oxidation involved in NAD biosynthesis. Biochemistry 2005; 44: 7632-7643.
- Okada K, Angkawidjaja C, Koga Y, Kanaya S. Structural and mechanistic insights into the kynurenine aminotransferase-mediated excretion of kynurenic acid. J Struct Biol 2014; 185: 257-266.
- Okuno E, Tsujimoto M, Nakamura M, Kido R. 2-Aminoadipate-2-oxoglutarate aminotransferase isoenzymes in human liver: a plausible physiological role in lysine and tryptophan metabolism. Enzyme Protein 1993; 47: 136-148.
- Goh DL, Patel A, Thomas GH, et al. Characterization of the human gene encoding alpha-aminoadipate aminotransferase (AADAT). Mol Genet Metab 2002; 76: 172-180.
- Calderone V, Trabucco M, Menin V, Negro A, Zanotti G. Cloning of human 3-hydroxyanthranilic acid dioxygenase in Escherichia coli: characterisation of the purified enzyme and its in vitro inhibition by Zn(2+). Biochim Biophys Acta 2002; 1596: 283-292.
- Phillips RS, Scott I, Paulose R, Patel A, Barron TC. The phosphate of pyridoxal-5'-phosphate is an acid/base catalyst in the mechanism of Pseudomonas fluorescens kynureninase. FEBS J 2014; 281: 1100-1109.
- Stevens CO, Henderson LM. Riboflavin and hepatic kynurenine hydroxylase. J Biol Chem 1959; 234: 1191-1194.
- Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov 2002; 1: 6009-6020.
- Schartau W, Linzen B. The tryptophan 2,3-dioxygenase of the blowfly, Protophormia terrae-novae: partial purification and characterization. Hoppe Seylers Z Physiol Chem 1976; 357: 41-49.
- Serrano AE Jr, Nagayama F. Inhibition studies on liver arylformamidases of rainbow trout and cattle. Comp Biochem Physiol B 1991; 99: 281-285.
- Pucci L, Perozzi S, Cimadamore F, Orsomando G, Raffaelli N. Tissue expression and biochemical characterization of human 2-amino 3-carboxymuconate 6-semialdehyde decarboxylase, a key enzyme in tryptophan catabolism. FEBS J 2007; 274: 827-840.
- Garavaglia S, Perozzi S, Galeazzi L, Raffaelli N, Rizzi M. The crystal structure of human alpha-amino-beta-carboxymuconate-epsilon-semialdehyde decarboxylase in complex with 1,3-dihydroxyacetonephosphate suggests a regulatory link between NAD synthesis and glycolysis. FEBS J 2009; 276: 6615-6623.
- Liu H, Woznica K, Catton G, Crawford A, Botting N, Naismith JH. Structural and kinetic characterization of quinolinate phosphoribosyltransferase (hQPRTase) from homo sapiens. J Mol Biol 2007; 373: 755-763.
- Shibata K, Iwai K. Isolation and properties of crystalline quinolinate phosphoribosyltransferase from hog kidney. Biochim Biophys Acta 1980; 611: 280-288.
- Iwai K, Taguchi H. Crystallization and properties of quinolinate phosphoribosyltransferase from hog liver. Methods Enzymol 1980; 66: 96-101.
- Huo L, Liu F, Iwaki H, Li T, Hasegawa Y, Liu A. Human α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase (ACMSD): a structural and mechanistic unveiling. Proteins 2015; 83: 178-187.
- Capece L, Lewis-Ballester A, Yeh SR, Estrin DA, Marti MA. Complete reaction mechanism of indoleamine 2,3-dioxygenase as revealed by QM/MM simulations. J Phys Chem B 2012; 116: 1401-1413.
- Oxenkrug G. Tryptophan-kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: serotonin hypothesis revisited 40 years later. Isr J Psychiatry Relat Sci 2010; 47: 56-63.
- Blankfield AA. Brief historic overview of clinical disorders associated with tryptophan: the relevance to chronic fatigue syndrome (CFS) and fibromyalgia (FM). Int J Tryptophan Res 2012; 5: 27-32.
- Feria-Velasco A, Mena-Munguia S, Carabez-Torres J, et al. Low tryptophan and protein in the diet during development increase the susceptibility to convulsions in adult rats. Neurochem Res 2008; 33: 1484-1491.
- Castrogiovanni P, Musumeci G, Trovato FM, Avola R, Magro G, Imbesi R. Effects of high-tryptophan diet on pre- and postnatal development in rats: a morphological study. Eur J Nutr 2014; 53: 297-308.
- Strasser B, Berger K, Fuchs D. Effects of a caloric restriction weight loss diet on tryptophan metabolism and inflammatory biomarkers in overweight adults. Eur J Nutr 2015; 54: 101-107.
- Hansen MB, Witte AB. The role of serotonin in intestinal luminal sensing and secretion. Acta Physiol (Oxf) 2008; 193: 311-323.
- Guseva D, Holst K, Kaune B, et al. Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract. Inflamm Bowel Dis 2014; 20: 1516-1529.
- Stepien A, Walecka-Kapica E, Blonska A, Klupinska G. The role of tryptophan and serotonin in pathogenesis and treatment of irritable bowel syndrome. Folia Medica Lodziensia 2014; 41: 139-154.
- Kim JJ, Khan WI. 5-HT7 receptor signalling: improved therapeutic strategy in gut disorders. Front Behav Neurosci 2014; 8: 396. doi: 10.3389/fnbeh.2014.00396.
- Paul-Savoie E, Potvin S, Daigle K. A deficit in peripheral serotonin levels in major depressive disorder but not in chronic widespread pain. Clin J Pain 2011; 27: 529-534.
- Mestre TA, Zurowski M, Fox SH. 5-Hydroxytryptamine 2A receptor antagonists as potential treatment for psychiatric disorders. Expert Opin Investig Drugs 2013; 22: 411-421.
- Garvin B, Wiley JW. The role of serotonin in irritable bowel syndrome: implications for management. Curr Gastroenterol Rep 2008; 10: 363-368.
- Mitchell NA, Pepperell E, Ociepka S. 5-hydroxyindalpine, an agonist at the putative 5-HT receptor, has no activity on human recombinant monoamine receptors but accelerates distension-induced peristalsis in mouse isolated colon. Neurogastroenterol Motil 2009; 21: 760-e48. doi: 10.1111/j.1365-2982.2009.01275.x
- Michel K, Sann H, Schaaf C, Schemann M. Subpopulations of gastric myenteric neurons are differentially activated via distinct serotonin receptors: projection, neurochemical coding, and functional implications. J Neurosci 1997; 17: 8009-8017.
- Branchek TA, Mawe GM, Gershon MD. Characterization and localization of a peripheral neural 5-hydroxytryptamine receptor subtype (5-HT1P) with a selective agonist, 3H-5-hydroxyindalpine. J Neurosci 1988; 8: 2582-2595.
- Mawe GM, Branchek TA, Gershon MD. Peripheral neural serotonin receptors: identification and characterization with specific antagonists and agonists. Proc Natl Acad Sci USA 1986; 83: 9799-9803.
- Gershon MD, Wade PR, Fiorica-Howells E, Kirchgessner AL, Tamir H. 5-HT1P receptors in the bowel: G protein coupling, localization, and function. In: Serotonin: Molecular Biology, Receptors and Functional Effects. Fozard JR, Saxena PR (eds). Basel, Birkhauser, 1991, pp 133-143.
- Cohen ML, Fludzinski LA. Contractile serotonergic receptor in rat stomach fundus. J Pharmacol Exp Ther 1987; 243: 264-269.
- Borman RA, Burleigh DE. Functional evidence for a 5-HT2B receptor mediating contraction of longitudinal muscle in human small intestine. Br J Pharmacol 1995; 114: 1525-1527.
- Borman RA, Tilford NS, Harmer DW. 5-HT(2B) receptors play a key role in mediating the excitatory effects of 5-HT in human colon in vitro. Br J Pharmacol 2002; 135: 1144-1151.
- McLean PG, Coupar IM. Characterisation of a postjunctional 5-HT7-like and a prejunctional 5-HT3 receptor mediating contraction of rat isolated jejunum. Eur J Pharmacol 1996; 312: 215-225.
- Beutler E. Serotonin as an intestinal secretagogue. In: Serotonin and Gastrointestinal Function. Gaginella TS, Galligan JJ (eds). Boca Raton, CRC Press, 1995, pp. 85-101.
- Buchheit KH, Engel G, Mutschler E, Richardson B. Study of the contractile effect of 5-hydroxytryptamine (5-HT) in the isolated longitudinal muscle strip from guinea-pig ileum. Evidence for two distinct release mechanisms. Naunyn Schmiedebergs Arch Pharmacol 1985; 329: 36-41.
- Gore S, Gilmore IT, Haigh CG, Brownless SM, Stockdale H, Morris AI. Colonic transit in man is slowed by ondansetron (GR38032F), a selective 5-hydroxytryptamine receptor (type 3) antagonist. Aliment Pharmacol Ther 1990; 4: 139-144.
- Tyers MB, Freeman AJ. Mechanism of the anti-emetic activity of 5-HT3 receptor antagonists. Oncology 1992; 49: 263-268.
- Budhoo MR, Harris RP, Kellum JM. The role of the 5-HT4 receptor in Cl- secretion in human jejunal mucosa. Eur J Pharmacol 1996; 314: 109-114.
- Kuemmerle JF, Murthy KS, Grider JR, Martin DC, Makhlouf GM. Coexpression of 5-HT2A and 5-HT4 receptors coupled to distinct signaling pathways in human intestinal muscle cells. Gastroenterology 1995; 109: 1791-1800.
- Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci 2009; 29: 9683-9699.
- Del Tredici K, Jost WH. Gastrointestinal dysfunction in idiopathic Parkinson's disease. Nervenarzt 2012; 83: 1282-1291.
- Takaki M, Goto K, Kawahara I. The 5-hydroxytryptamine 4 receptor agonist-induced actions and enteric neurogenesis in the gut. J Neurogastroenterol Motil 2014; 20: 17-30.
- Carter D, Champney M, Hwang B, Eglen RM. Characterization of post-junctional 5-hydroxytryptamine (5-HT) receptor mediating relaxation of guinea-pig isolated ileum. Eur J Pharmacol 1995; 280: 243-250.
- Briejer MR, Akkermans LM, Meulemans AL, Lefebvre RA, Schuurkes JA. Nitric oxide is involved in 5-HT-induced relaxations of the guinea-pig colon ascendens in vitro. Br J Pharmacol 1992; 107: 756-761.
- Elswood CJ, Bunce KT. Investigation of the 5-HT receptor mediating relaxation in guinea-pig proximal colon. J Pharm Pharmacol 1992; 44: 264-266.
- Krobert KA, Bach T, Syversveen T, Kvingedal AM, Levy FO. The cloned human 5-HT7 receptor splice variants: a comparative characterization of their pharmacology, function and distribution. Naunyn Schmiedebergs Arch Pharmacol 2001; 363: 620-632.
- Prins NH, Briejer MR, Van Bergen PJ, Akkermans LM, Schuurkes JA. Evidence for 5-HT7 receptors mediating relaxation of human colonic circular smooth muscle. Br J Pharmacol 1999; 128: 849-852.
- Galano A, Tan DX, Reiter RJ. On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J Pineal Res 2013; 54: 245-257.
- Reiter RJ, Tan DX, Rosales-Corral S, Manchester LC. The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini Rev Med Chem 2013; 13: 373-384.
- Mao L, Cheng Q, Guardiola-Lemaitre B, et al. in vitro and in vivo antitumor activity of melatonin receptor agonists. J Pineal Res 2010; 49: 210-221.
- Guo P, Pi H, Xu S, et al. Melatonin improves mitochondrial function by promoting MT1/SIRT1/PGC-1 alpha-dependent mitochondrial biogenesis in cadmium-induced hepatotoxicity in vitro. Toxicol Sci 2014; 142: 182-195.
- Campbell BM, Charych E, Lee AW, Moller T. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci 2014; 6: 8-12.
- Amori L, Guidetti P, Pellicciari R, Kajii Y, Schwarcz R. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J Neurochem 2009; 109: 316-325.
- Adams S, Teo C, McDonald KL. Involvement of the kynurenine pathway in human glioma pathophysiology. PLoS One 2014; 9: e112945. doi: 10.1371/journal.pone.0112945
- Reyes Ocampo J, Lugo Huitron R, Gonzalez-Esquivel D, et al. Kynurenines with neuroactive and redox properties: relevance to aging and brain diseases. Oxid Med Cell Longev 2014; 2014: 646909. doi: 10.1155/2014/646909.
- Peters JC. Tryptophan nutrition and metabolism: an overview. Adv Exp Med Biol 1991; 294: 345-358.
- Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res 2009; 2: 1-19.
- Lugo Huitron R, Ugalde Muniz P, Pineda B, Pedraza-Chaverri J, Rios C, Perez-de la Cruz V. Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxid Med Cell Longev 2013; 2013: 104024. doi: 10.1155/2013/104024
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci 2001; 21: 7463-7473.
- Widner B, Leblhuber F, Walli J, Tilz GP, Demel U, Fuchs D. Tryptophan degradation and immune activation in Alzheimer's disease. J Neural Transm (Vienna) 2000; 107: 343-353.
- DeMyer MK, Shea PA, Hendrie HC, Yoshimura NN. Plasma tryptophan and five other amino acids in depressed and normal subjects. Arch Gen Psychiatry 1981; 38: 642-646.
- Joseph MS, Brewerton TD, Reus VI, Stebbins GT. Plasma L-tryptophan/neutral amino acid ratio and dexamethasone suppression in depression. Psychiatry Res 1984; 11: 185-192.
- Maes M, Verkerk R, Bonaccorso S, Ombelet W, Bosmans E, Scharpe S. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci 2002; 71: 1837-1848.
- Maes M, Ombelet W, Verkerk R, Bosmans E, Scharpe S. Effects of pregnancy and delivery on the availability of plasma tryptophan to the brain: relationships to delivery-induced immune activation and early post-partum anxiety and depression. Psychol Med 2001; 31: 847-858.
- Dursun SM, Farrar G, Handley SL, Rickards H, Betts T, Corbett JA. Elevated plasma kynurenine in Tourette syndrome. Mol Chem Neuropathol 1994; 21: 55-60.
- Sathyasaikumar KV, Stachowski EK, Wonodi I, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull 2011; 37: 1147-1156.
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 2001; 50: 521-530.
- Logters TT, Laryea MD, Altricher J, et al. Increased plasma kynurenine values and kynurenine-tryptophan ratios after major trauma are early indicators for the development of sepsis. Shock 2009; 32: 29-34.
- van de Kamp JL, Smolen A. Response of kynurenine pathway enzymes to pregnancy and dietary level of vitamin B-6. Pharmacol Biochem Behav 1995; 51: 753-758.
- Wachstein M. Evidence for abnormal vitamin B6 metabolism in pregnancy and various disease states. Am J Clin Nutr 1956; 4: 369-373.
- Schwarz MJ, Guillemin GJ, Teipel SJ, Buerger K, Hampel H. Increased 3-hydroxykynurenine serum concentrations differentiate Alzheimer's disease patients from controls. Eur Arch Psychiatry Clin Neurosci 2013; 263: 345-352.
- Yan EB, Frugier T, Lim CK, et al. Activation of the kynurenine pathway and increased production of the excitotoxin quinolinic acid following traumatic brain injury in humans. J Neuroinflammation 2015; 12: 110. doi: 10.1186/s12974-015-0328-2
- Eussen SJ, Ueland PM, Vollset SE, et al. Kynurenines as predictors of acute coronary events in the Hordaland Health Study. Int J Cardiol 2015; 189: 18-24.
- Guidetti P, Luthi-Carter RE, Augood SJ, Schwarcz R. Neostriatal and cortical quinolinate levels are increased in early grade Huntington's disease. Neurobiol Dis 2004; 17: 455-461.
- Ogawa T, Matson WR, Beal MF, et al. Kynurenine pathway abnormalities in Parkinson's disease. Neurology 1992; 42: 1702-1706.
- Lewitt PA, Li J, Lu M, et al. 3-hydroxykynurenine and other Parkinson's disease biomarkers discovered by metabolomic analysis. Mov Disord 2013; 28: 1653-1660.
- Bender DA, Njagi EN, Danielian PS. Tryptophan metabolism in vitamin B6-deficient mice. Br J Nutr 1990; 63: 27-36.
- Stoy N, Mackay GM, Forrest CM, et al. Tryptophan metabolism and oxidative stress in patients with Huntington's disease. J Neurochem 2005; 93: 611-623.
- Deac OM, Mills JL, Shane B, et al. Tryptophan catabolism and vitamin B-6 status are affected by gender and lifestyle factors in healthy young adults. J Nutr 2015; 145: 701-707.
- Baran H, Jellinger K, Deecke L. Kynurenine metabolism in Alzheimer's disease. J Neural Transm (Vienne) 1999; 106: 165-181.
- Oxenkrug GF. Increased plasma levels of xanthurenic and kynurenic acids in type 2 diabetes. Mol Neurobiol 2015; 52: 805-810.
- Oxenkrug G, Ratner R, Summergrad P. Kynurenines and vitamin B6: link between diabetes and depression. J Bioinform Diabetes 2013; 1: pii: http://openaccesspub.org/journals/ download.php?file=51-OAP-JBD-IssuePDF.pdf.
- Oxenkrug G, Tucker KL, Requintina P, Summergrad P. Neopterin, a marker of interferon-gamma-inducible inflammation, correlates with pyridoxal-5'-phosphate, waist circumference, HDL-cholesterol, insulin resistance and mortality risk in adult Boston community dwellers of Puerto Rican origin. Am J Neuroprot Neuroregen 2011; 3: 48-52.
- Oxenkrug G. Insulin resistance and dysregulation of tryptophan - kynurenine and kynurenine - nicotinamide adenine dinucleotide metabolic pathways. Mol Neurobiol 2013; 48: 294-301.
- Heyes MP, Saito K, Crowley JS, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain 1992; 115: 1249-1273.
- Hartai Z, Juhasz A, Rimanoczy A, et al. Decreased serum and red blood cell kynurenic acid levels in Alzheimer's disease. Neurochem Int 2007; 50: 308-313.
- Jauch D, Urbanska EM, Guidetti P, et al. Dysfunction of brain kynurenic acid metabolism in Huntington's disease: focus on kynurenine aminotransferases. J Neurol Sci 1995; 130: 39-47.
- Watanabe M. Microanalysis of tryptophan metabolites and suppressor factor of delayed-type hypersensitivity in mice. Yakugaku Zasshi 2002; 122: 429-434.
- Fujii K. Evaluation of the newborn mouse model for chemical tumorigenesis. Carcinogenesis 1991; 12: 1409-1415.
- Chobot V, Hadacek F, Weckwerth W, Kubicova L. Iron chelation and redox chemistry of anthranilic acid and 3-hydroxyanthranilic acid: a comparison of two structurally related kynurenine pathway metabolites to obtain improved insights into their potential role in neurological disease development. J Organomet Chem 2015; 782: 103-110.
- Miu J, Ball HJ, Mellor AL, Hunt NH. Effect of indoleamine dioxygenase-1 deficiency and kynurenine pathway inhibition on murine cerebral malaria. Int J Parasitol 2009; 39: 363-370.
- Ting KK, Brew BJ, Guillemin GJ. Effect of quinolinic acid on human astrocytes morphology and functions: implications in Alzheimer's disease. J Neuroinflammation 2009; 36: 1-13.
- Sadan O, Shemesh N, Barzilay R, et al. Mesenchymal stem cells induced to secrete neurotrophic factors attenuate quinolinic acid toxicity: a potential therapy for Huntington's disease. Exp Neurol 2012; 234: 417-427.
- Comai S, Costa CVL, Ragazzi E, Bertazzo A, Allegri G. The effect of age on the enzyme activities of tryptophan metabolism along the kynurenine pathway in rats. Clinica Chimica Acta 2005; 360: 67-80.
- Moroni F, Lombardi G, Moneti G, Aldinio C. The excitotoxin quinolinic acid is present in the brain of several mammals and its cortical content increases during the aging process. Neurosci Lett 1984; 47: 51-55.
- Grimaldi BL. The central role of magnesium deficiency in Tourette's syndrome: causal relationships between magnesium deficiency, altered biochemical pathways and symptoms relating to Tourette's syndrome and several reported comorbid conditions. Med Hypotheses 2002; 58: 47-60.
- Sasaki N, Egashira Y, Sanada H. Production of L-tryptophan-derived catabolites in hepatocytes from streptozotocin-induced diabetic rats. Eur J Nutr 2009; 48: 145-153.
- Matsuda H, Sato M, Yakushiji M, Koshiguchi M, Hirai S, Egashira Y. Regulation of rat hepatic a-amino-b-carboxymuconate-e-semialdehyde decarboxylase, a key enzyme in the tryptophan- NAD pathway, by dietary cholesterol and sterol regulatory element-binding protein-2. Eur J Nutr 2014; 53: 469-477.
- Kohlmeier M. Nutrient Metabolism: Structures, Functions, and Genetics. In Food Science and Technology, London, Academic Press/Elsevier Press, 2003, pp. 328-338.
- Shibata K, Hirose J, Fukuwatari T. Method for evaluation of the requirements of B-group vitamins using tryptophan metabolites in human urine. Int J Tryptophan Res 2015; 8: 31-39.
- Pedersen ER, Tuseth N, Eussen SJ, et al. Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol 2015; 35: 455-462.
- Muller AJ, DuHadaway JB, Chang MY, et al. Non-hematopoietic expression of IDO is integrally required for inflammatory tumor promotion. Cancer Immunol Immunother 2010; 59: 1655-1663.
- Silk JD, Lakhal S, Laynes R. Indoleamine 2,3-dioxygenase induces expression of a novel tryptophan transporter in mouse and human tumor cells. J Immunol 2011; 187: 1617-1625.
- Clarke G, Fitzgerald P, Cryan JF, Cassidy EM, Quigley EM, Dinan TG. Tryptophan degradation in irritable bowel syndrome: evidence of indoleamine 2,3-dioxygenase activation in a male cohort. BMC Gastroenterol 2009; 9: 6. doi: 10.1186/1471-230X-9-6.
- Fitzgerald P, Cassidy EM, Clarke G, et al. Tryptophan catabolism in females with irritable bowel syndrome: relationship to interferon-gamma, severity of symptoms and psychiatric co-morbidity. Neurogastroenterol Motil 2008; 20: 1291-1297.
- Giorgini F, Guidetti P, Nguyen QV, Bennett SC, Muchowski PJ. A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington's disease. Nat Genet 2005; 37: 526-531.
- Toledo-Sherman LM, Prime ME, Mrzljak L, et al. Development of a series of aryl pyrimidine kynurenine monooxygenase inhibitors as potential therapeutic agents for the treatment of Huntington's disease. J Med Chem 2015; 58: 1159-1183.
- Jin H, Zhang Y, You H, et al. Prognostic significance of kynurenine 3-monooxygenase and effects on proliferation, migration, and invasion of human hepatocellular carcinoma. Sci Rep 2015; 5: 10466. doi: 10.1038/srep10466.
- Rossi F, Schwarcz R, Rizzi M. Curiosity to kill the KAT (kynurenine aminotransferase): structural insights into brain kynurenic acid synthesis. Curr Opin Struct Biol 2008; 18: 748-755.
- Jayawickrama GS, Sadig RR, Sun G, et al. Kynurenine aminotransferases and the prospects of inhibitors for the treatment of schizophrenia. Curr Med Chem 2015; 22: 2902-2918.
- Mackay GM, Forrest CM, Stoy N, et al. Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Eur J Neurol 2006; 13: 30-42.
- Darlington LG, Mackay GM, Forrest CM, Stoy N, George C, Stone TW. Altered kynurenine metabolism correlates with infarct volume in stroke. Eur J Neurosci 2007; 26: 2211-2221.
- Mackay GM, Forrest CM, Christofides J, et al. Kynurenine metabolites and inflammation markers in depressed patients treated with fluoxetine or counselling. Clin Exp Pharmacol Physiol 2008; 36: 425-35.
- Forrest CM, Mackay GM, Oxford L, Stoy N, Stone TW, Darlington LG. Kynurenine pathway metabolism in patients with osteoporosis after 2 years of drug treatment. Clin Exp Pharmacol Physiol 2006; 33: 1078-1087.
- Aitken JB, Austin CJ, Hunt NH, Ball HJ, Lay PA. The Fe-heme structure of metindoleamine 2,3-dioxygenase-2 determined by X-ray absorption fine structure. Biochem Biophys Res Commun 2014; 450: 25-29.
- Ball HJ, Sanchez-Perez A, Weiser S, et al. Characterization of an indoleamine 2, 3-dioxygenase-like protein found in humans and mice. Gene 2007; 396: 203-213.
- Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun 2010; 24: 9-16.
- Li JS, Han Q, Fang J, Rizzi M, James AA, Li J. Biochemical mechanisms leading to tryptophan 2,3-dioxygenase activation. Arch Insect Biochem Physiol 2007; 64: 74-87.
- Midttun O, Ulvik A, Pedersen E, et al. Low plasma vitamin B-6 status affects metabolism through the kynurenine pathway in cardiovascular patients with systemic inflammation. J Nutr 2011; 141: 611-617.
- Metz R, Duhadaway JB, Kamasani U, et al. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res 2007; 67: 7082-7087.
- Metz R, Smith C, DuHadaway JB. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int Immunol 2014; 26: 357-367.
- Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res 2012; 72: 5435-5440.
- Hansen AM, Ball HJ, Mitchell AJ, Miu J, Takikawa O, Hunt NH. Increased expression of indoleamine 2,3-dioxygenase in murine malaria infection is predominantly localised to the vascular endothelium. Int J Parasitol 2004; 34: 1309-1319.
- Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011; 478: 197-203.
- Wichers MC, Maes M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-a-induced depression. J Psychiatry Neurosci 2004; 29: 11-17.
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry 1995; 19: 11-38.
- Clarke G, McKernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG. A distinct profile of tryptophan metabolism along the kynurenine pathway downstream of toll-like receptor activation in irritable bowel syndrome. Front Pharmacol 2012; 3: 90. doi: 10.3389/fphar.2012.00090.
- Chiarugi A, Dello Sbarba P, Paccagnini A, Donnini S, Filippi S, Moroni F. Combined inhibition of indoleamine 2,3-dioxygenase and nitric oxide synthase modulates neurotoxin release by interferon-gamma-activated macrophages. J Leukoc Biol 2000; 68: 260-266.
- Okuda S, Nishiyama N, Saito H, Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J Neurochem 1998; 70: 299-307.
- Crozier-Reabe KR, Phillips RS, Moran GR. Kynurenine 3-monooxygenase from Pseudomonas fluorescens: substrate-like inhibitors both stimulate flavin reduction and stabilize the flavin-peroxo intermediate yet result in the production of hydrogen peroxide. Biochemistry 2008; 47: 12420-12433.
- Wilson K, Mole DJ, Binnie M, et al. Bacterial expression of human kynurenine 3-monooxygenase: solubility, activity, purification. Protein Expr Purif 2014; 95: 96-103.
- Okamoto H, Yamamoto S, Nozaki M, Hayaishi O. On the submitochondrial localization of L-kynurenine-3-hydroxylase. Biochem Biophys Res Commun 1967; 26: 309-314.
- Uemura T, Hirai K. L-kynurenine 3-monooxygenase from mitochondrial outer membrane of pig liver: purification, some properties and monoclonal antibodies directed to the enzyme. J Biochem 1998; 123: 253-262.
- Zwilling D, Huang SY, Sathyasaikumar KV, et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell 2011; 145: 863-874.
- Moroni F, Cozzi A, Carpendo R, Cipriani G, Veneroni O, Izzo E. Kynurenine 3-mono-oxygenase inhibitors reduce glutamate concentration in the extracellular spaces of the basal ganglia but not in those of the cortex or hippocampus. Neuropharmacology 2005; 48: 788-795.
- Samadi P, Gregoire L, Rassoulpour A, et al. Effect of kynurenine 3-hydroxylase inhibition on the dyskinetic and antiparkinsonian responses to levodopa in Parkinsonian monkeys. Mov Disord 2005; 20: 792-802.
- Phillips RS. Structure and mechanism of kynureninase. Arch Biochem Biophys 2014; 544: 69-74.
- Takeuchi F, Tsubouchi R, Izuta S, Shibata Y. Kynurenine metabolism and xanthurenic acid formation in PLP-deficient rat after tryptophan injection. J Nutr Sci Vitaminol 1989; 35: 111-122.
- Rios-Avila L, Nijhout HF, Reed MC, Sitren HS, Gregory JF. A mathematical model of tryptophan metabolism via the kynurenine pathway provides insights into the effects of vitamin B-6 deficiency, tryptophan loading, and induction of tryptophan 2,3-dioxygenase on tryptophan metabolites. J Nutr 2013; 143: 1509-1519.
- Donald EA, Bosse TR. The PLP requirement in oral contraceptive users. II. Assessment by tryptophan metabolites, PLP, and pyridoxic acid levels in urine. Am J Clin Nutr 1979; 32: 1024-1032.
- Leklem JE, Brown RR, Rose DP, Linkswiler H, Arend RA. Metabolism of tryptophan and niacin in oral contraceptives users receiving controlled intakes of PLP. Am J Clin Nutr 1975; 28: 146-156.
- Yess N, Price JM, Brown RR, Swan PB, Linkswiler H. PLP depletion in man: urinary excretion of quinolinic acid and niacin metabolites. J Nutr 1965; 87: 419-423.
- Yeh JK, Brown RR. Effects of vitamin B-6 deficiency and tryptophan loading on urinary excretion of tryptophan metabolites in mammals. J Nutr 1977; 107: 261-271.
- Ulvik A, Theofylaktopoulou D, Midttun O, Nygard O, Eussen S, Ueland PM. Substrate product ratios of enzymes in the kynurenine pathway measured in plasma as indicators of functional vitamin B-6 status. Am J Clin Nutr 2013; 98: 934-940.
- Okuda S, Nishiyama N, Saito H, Katsuki H. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proc Natl Acad Sci USA 1996; 93: 12553-12558.
- Moriguchi M, Yamamoto T, Soda K. Inactivation of kynureninase by L-alanine. Biochem Biophys Res Commun 1971; 44: 1416-1419.
- Carpenedo R, Chiarugi A, Russi P. Inhibitors of kynurenine hydroxylase and kynureninase increase cerebral formation of kynurenate and have sedative and anticonvulsant activities. Neuroscience 1994; 61: 237-243.
- Bellocchi D, Macchiarulo A, Carotti A, Pellicciari R. Quantum mechanics/molecular mechanics (QM/MM) modeling of the irreversible transamination of L-kynurenine to kynurenic acid: the round dance of kynurenine aminotransferase II. Biochim Biophys Acta 2009; 1794: 1802-1812.
- Konradsson-Geuken A, Wu HQ, Gash CR, et al. Cortical kynurenic acid bi-directionally modulates prefrontal glutamate levels as assessed by microdialysis and rapid electrochemistry. Neuroscience 2010; 169; 848-1859.
- Wu HQ, Pereira EF, Bruno JP, Pellicciari R, Albuquerque EX, Schwarcz R. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J Mol Neurosci 2010; 40: 204-210.
- Pawley AC, Flesher S, Boegman RJ, Beninger RJ, Jhamandas KH. Differential action of NMDA antagonists on cholinergic neurotoxicity produced by N-methyl-D-aspartate and quinolinic acid. Br J Pharmacol 1996; 117: 1059-1064.
- Han Q, Beerntsen BT, Li J. The tryptophan oxidation pathway in mosquitoes with emphasis on xanthurenic acid biosynthesis. J Insect Physiol 2007; 53: 254-263.
- Han Q, Li J, Li J. pH Dependence, substrate specificity and inhibition of human kynurenine aminotransferase I. Eur J Biochem 2004; 271: 4804-4814.
- Baran H, Amann G, Lubec B, Lubec G. Kynurenic acid and kynurenine aminotransferase in heart. Pediatr Res 1997; 41: 404-410.
- Koshy Cherian A, Gritton H, Johnson DE, Young D, Kozak R, Sarter M. A systemically-available kynurenine aminotransferase II (KAT-II) inhibitor restores nicotine-evoked glutamatergic activity in the cortex of rats. Neuropharmacology 2014; 82: 41-48.
- Pellicciari R, Rizzo RC, Costantino G, et al. Modulators of the kynurenine pathway of tryptophan metabolism: synthesis and preliminary biological evaluation of (S)-4-(ethylsulfonyl)benzoylalanine, a potent and selective kynurenine aminotransferase II (KAT II) inhibitor. ChemMedChem 2006; 1: 528-531.
- Wennstrom M, Nielsen HM, Orhan F, Londos E, Minthon L, Erhardt S. Kynurenic acid levels in cerebrospinal fluid from patients with Alzheimer's disease or dementia with lewy bodies. Int J Tryptophan Res 2014; 7: 1-7.
- Erhardt S, Schwieler L, Nilsson L, Linderholm K, Engberg G. The kynurenic acid hypothesis of schizophrenia. Physiol Behav 2007; 92: 203-209.
- Mo X, Pi L, Yang J, Xiang Z, Tang A. Serum indoleamine 2,3-dioxygenase and kynurenine aminotransferase enzyme activity in patients with ischemic stroke. J Clin Neurosci 2014; 21: 482-486.
- Kocki T, Luchowski P, Luchowska E, Wielosz M, Turski WA, Urbanska EM. L-cysteine sulphinate, endogenous sulphur-containing amino acid, inhibits rat brain kynurenic acid production via selective interference with kynurenine aminotransferase II. Neurosci Lett 2003; 346: 97-100.
- Copeland CS, Neale SA, Salt TE. Actions of xanthurenic acid, a putative endogenous Group II metabotropic glutamate receptor agonist, on sensory transmission in the thalamus. Neuropharmacology 2013; 66: 133-142.
- Gobaille S, Kemmel V, Brumaru D, Dugave C, Aunis D, Maitre M. Xanthurenic acid distribution, transport, accumulation and release in the rat brain. J Neurochem 2008; 105: 982-993.
- Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther 2002; 303: 1-10.
- Nandi D, Lightcap ES, Koo YK, Lu X, Quancard J, Silverman RB. Purification and inactivation of 3-hydroxyanthranilic acid 3,4-dioxygenase from beef liver. Int J Biochem Cell Biol 2003; 35: 1085-1097.
- Sahm F, Oezen I, Opitz CA, et al. The endogenous tryptophan metabolite and NAD+ precursor quinolinic acid confers resistance of gliomas to oxidative stress. Cancer Res 2013; 73: 3225-3234.
- Foster AC, White RJ, Schwarcz R. Synthesis of quinolinic acid by 3-hydroxyanthranilic acid oxygenase in rat brain tissue in vitro. J Neurochem 1986; 47: 23-30.
- Krause D, Suh HS, Tarassishin L. The tryptophan metabolite 3-hydroxyanthranilic acid plays anti-inflammatory and neuroprotective roles during inflammation: role of hemeoxygenase-1. Am J Pathol 2011; 179: 1360-1372.
- Darlington LG, Forrest CM, Mackay GM, et al. On the biological importance of the 3-hydroxyanthranilic acid: anthranilic acid ratio. Int J Tryptophan Res 2010; 3: 51-59.
- Stachowski EK, Schwarcz R. Regulation of quinolinic acid neosynthesis in mouse, rat and human brain by iron and iron chelators in vitro. J Neural Transm 2012; 119: 123-131.
- Jhamandas KH, Boegman RJ, Beninger RJ, Miranda AF, Lipic KA. Excitotoxicity of quinolinic acid: modulation by endogenous antagonists. Neurotox Res 2000; 2: 139-155.
- Foster AC, Zinkand WC, Schwarcz R. Quinolinic acid phosphoribosyltransferase in rat brain. J Neurochem 1985; 44: 446-454.
- Foster AC, Whetsell WO, Bird ED, Schwarcz R. Quinolinic acid phosphoribosyltransferase in human and rat brain: activity in Huntington's disease and in quinolinatelesioned rat striatum. Brain Res 1985; 336: 207-214.
- Weidele K, Kunzmann A, Schmitz M, Beneke S, Burkle A. Ex vivo supplementation with nicotinic acid enhances cellular poly(ADP-ribosyl)ation and improves cell viability in human peripheral blood mononuclear cells. Biochem Pharmacol 2010; 80: 1103-1112.
- Stipek S, Stastny F, Platenik J, Crkovska J, Zima T. The effect of quinolinate on rat brain lipid peroxidation is dependent on iron. Neurochem Int 1997; 30: 233-237.
- Bohar Z, Toldi J, Fulop F, Vecsei L. Changing the face of kynurenines and neurotoxicity: therapeutic considerations. Int J Mol Sci 2015; 16: 9772-9793.
- Celinski K, Konturek PC, Slomka M, et al. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease--14 months follow up. J Physiol Pharmacol 2014; 65: 75-82.
- Shabbir F, Patel A, Mattison C, et al. Effect of diet on serotonergic neurotransmission in depression. Neurochem Int 2013; 62: 324-329.
- Kaneko I, Sabir MS, Dussik CM, et al. 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: implication for behavioral influences of vitamin D. FASEB J 2015; 29: 4023-4035.
- Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J 2014; 28: 2398-2413.
- Zagajewski J, Drozdowicz D, Brzozowska I, et al. Conversion L-tryptophan to melatonin in the gastrointestinal tract: the new high performance liquid chromatography method enabling simultaneous determination of six metabolites of L-tryptophan by native fluorescence and UV-VIS detection. J Physiol Pharmacol 2012; 63: 613-621.
- Majewski M, Kozlowska A, Thoene M, Lebiedzinska A. Variations of niacin content with regard to carbohydrates in energy-rich diets of elite European athletes and their relation with dietary RDA. J Elem 2016; 21(3). doi: 10.5601/jelem.2015.20.4.921
- Zayachkivska O, Pshyk-Titko I, Hrycevych N, Savytska M. New insight into oseophageal injury and protection in physiologically relevant animal models. J Physiol Pharmacol 2014; 65: 295-307.
A c c e p t e d : December 9, 2015