BI-DIRECTIONAL CB1 RECEPTOR-MEDIATED CARDIOVASCULAR
EFFECTS OF CANNABINOIDS IN ANAESTHETIZED RATS:
ROLE OF THE PARAVENTRICULAR NUCLEUS
INTRODUCTION
Cannabinoids induce complex cardiovascular changes by acting directly or indirectly via various peripheral and/or central receptors: cannabinoid CB1 and CB2, vanilloid TRPV1, O-1918-sensitive endothelial or GPR18 cannabinoid receptors as well as thromboxane A2 (TP), N-methyl-D-aspartate (NMDA) and β2-adrenergic receptors (1-4). In humans cannabis preparations including their major psychotropic constituent Δ9-tetrahydrocannabinol (Δ9-THC) evoke a rapid increase in heart rate (HR), which may be accompanied by a modest increase in blood pressure (BP) (1, 4).
The cardiovascular effects of cannabinoids differ between anaesthetized and conscious animals. Thus, in urethane-anaesthetized rats or pentobarbitone-anaesthetized mice, a prolonged decrease in BP accompanied by a decrease in HR (the so-called phase III) is the most prominent effect of intravenous (i.v.) cannabinoid administration [including Δ9-THC, the endocannabinoid anandamide (AEA), its synthetic analogue methanandamide (MethAEA) or synthetic cannabinoid receptor agonists WIN55-212 or CP55940] (5-11). This effect follows phase I (a rapid and pronounced bradycardia and transient drop in BP) and phase II (a pressor response) induced by AEA, MethAEA and Δ9-THC (only phase II). On the contrary, in conscious rats the most distinct effect of AEA, MethAEA, Δ9-THC and WIN55212-2 is a pressor response (6, 12-14) (phase II). Phase I was also observed in response to higher doses AEA; however, unlike in anaesthetized rats, none of the five cannabinoids induced phase III.
Cannabinoid CB1 receptors have been proved (with the use of CB1 receptor antagonists or in CB1–/– mice) to be responsible for the prolonged hypotension in anaesthetized (phase III) and for the pressor response in conscious animals (phase II) (5-11). In the case of phase III, the CB1 receptors are located presynaptically on the sympathetic nerve endings innervating resistance vessels (15, 16) and heart (16, 17). For phase II, a central localization has been suggested. Thus, intracisternal injection of WIN55212-2 and CP55940 to conscious rabbits (18) and rats (19) increased BP, plasma noradrenaline levels and lowered HR in a manner sensitive to CB1 receptor antagonists. Interestingly, also central administration of cannabinoids induced a pressor effect in anaesthetized animals. Thus, injection of AEA, WIN55212-2 or HU-210 into the cisternal system (20), the rostral ventrolateral medulla (RVLM) (21) or the dorsal periaqueductal gray (dPAG) (22) of anaesthetized rats increased BP, plasma noradrenaline levels and/or renal sympathetic nerve activity. CB1 receptor antagonists diminished the above effects. Moreover, a pressor response to AEA i.v. was preceded by a transient rise in the activity of the RVLM and a brief rise in splanchnic sympathetic nerve discharge in urethane-anaesthetized rats (23).
It has been shown that in anaesthetized rats cannabinoids may also elicit pressor effects in a CB1 receptor-independent manner, do not elicit a pressor response at all or even lead to a depressor effect: (a) WIN55212-2 and CP55940 given i.v. did not increase BP (5); (b) cardiovascular effects of systemically administered Δ9-THC depend on the hindbrain and peripheral activation of cyclooxygenase (24); (c) the pressor response to AEA or MethAEA (i.v.) was not reduced by the CB1 receptor antagonist rimonabant (7); (d) intracerebroventricular (i.c.v.) injection of AEA and MethAEA increased BP only after the combined i.v. administration of a CB1 and TRPV1 receptor antagonist (8); (e) rimonabant given i.c.v. inhibited the endotoxic hypotension evoked by lipopolysaccharide in conscious and anaesthetized rats (25); (f) rimonabant given i.v. elicited a marked increase in BP (but not in HR) in anaesthetized hypertensive (but not normotensive) rats (26); this finding is very interesting in the light of the potential use of CB1 antagonists as antiobesity drugs since drugs increasing BP would create problems in patients suffering from a metabolic syndrome, i.e. a disorder combining increased body weight, high BP, raised fasting blood glucose and dyslipidemia (27).
The central regulation of cardiovascular functions depends upon neuroendocrine and autonomic nerve-mediated mechanisms in specific brain regions. One of them is the paraventricular nucleus of the hypothalamus (PVN), which acts as a major integrative site for metabolic, cardiovascular and respiratory functions (28). Cannabinoid CB1 receptors located in the PVN have been demonstrated to be involved in the regulation of food intake and energy homeostasis (29, 30), stress response (31) or penile erection (32). They may also play a role for the cardiovascular system since the hypertensive effect of angiotensin II microinjected into the PVN of anaesthetized rats is counteracted by the CB1 receptor antagonist AM251; this effect may be related to an increased formation of endogenous cannabinoids (33). Thus, the aims of our study were (a) to further determine the role of the PVN in the cardiovascular effects of cannabinoids in anaesthetized rats and (b) to check the involvement of CB1 receptors.
MATERIAL AND METHODS
Animals
Male Wistar normotensive rats (weighing 280 – 350 g) were used in the present experiments. All surgical procedures and experimental protocols were in accordance with European and Polish legislation and were approved by the local Animal Ethic Committee in Bialystok (Poland).
Mounting of cannulae for drug administration into the paraventricular nucleus of the hypothalamus
Rats were anaesthetized by intraperitoneal (i.p.) injection of pentobarbitone sodium (300 µmol/kg) and placed in a stereotaxic instrument (Stoelting WPI, Wood Dale, IL, USA). Stainless steel cannulae (outer and inner diameter of 0.5 and 0.3 mm, respectively) were stereotaxically implanted on the right side (bilaterally in the case of chemical lesion only), using established coordinates obtained from the Paxinos and Watson (34) rat brain atlas (1.5 mm caudal to the bregma; 0.5 mm lateral to the midline and 8 mm below the skull surface).
Anaesthetized rats
At least 7 days later rats were anaesthetized with urethane (14 mmol/kg; i.p.). The trachea was cannulated. The general procedure and the equipment for the measurement of mean BP (MBP), systolic BP (SBP), and diastolic BP (DBP) and heart rate (HR), the intravenous (i.v.; 0.5 ml/kg) and i.p. (2.0 ml/kg) drug administration, the vasopressin (VP) infusion and the maintenance of constant body temperature were like in our previous studies (e.g. 8, 10).
PVN microinjections were administered slowly in a volume of 100 nl per rat and completed within 1 min. Correct cannula placement was confirmed histologically and analyzed by light microscopy at the end of the experiment. Only animals for which the correct placement of the guide cannula to the PVN was confirmed were included in this study.
Experimental protocol
In most experiments MethAEA (8) or CP55940 (35) were administered into the PVN twice (S1 and S2, 20 min apart). The maximal changes in the particular cardiovascular parameters were recorded. Only one dose of MethAEA or CP55940 was examined in one rat. The CB1 receptor antagonist AM251 (3 µmol/kg) (8), the TRPV1 receptor antagonist ruthenium red (3 µmol/kg) (8), the CB2 receptor antagonist SR144528 (3 µmol/kg) (36) or their solvents were administered i.v. 5 min before S2. The peripherally restricted cannabinoid CB1 receptor antagonist AM6545 (15 µmol/kg, i.p) (37) was given 90 min before S1. In one series of experiments, AM251 (0.03 µmol/animal) (38) or its solvent was administered into the PVN during S2 together with CP55940 (0.1 nmol/animal) in a total volume of 100 nl.
In additional series of experiments, three increasing doses of CP55940 were administered i.v. with sufficient time for recovery to the preinjection value. The first dose of CP55940 was given 90 min after AM6545 (15 µmol/kg, i.p.) or its solvent or 90 min after chemical lesion of the PVN by bilateral PVN microinjection of kainic acid 2 nmol to each side (39) (in the corresponding control series, the solvent of kainic acid was used instead).
Preparation of paraventricular nucleus of the hypothalamus and cerebral cortex
At the end of the experiment, brains were quickly removed. The cortex and PVN were immediately dissected and analyzed. PVN punches were made from brain sections using a Stoelting brain punch with a diameter of 1.0 mm (Stoelting) according to Du et al. (40). The cortex and PVN were flash frozen in liquid nitrogen and stored at –80°C.
Measurement of anandamide levels
Anandamide was quantified according to the method described by Lam et al. (41) and modified by Marczylo et al. (42), using ultra high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). A Zorbax Extend C18 column (2.1 mm × 150 mm, 1.8 µm, Agilent, Santa Clara, CA, USA) was used and octadeuterated AEA (AEA-d8) served as internal standard. AEA has been isolated from brain tissue using a solid phase extraction method. UPLC-MS/MS analysis was performed using an Agilent 1290 UPLC system interfaced with an Agilent 6460 triple quadrupole mass spectrometer with electrospray ionization source. The samples were analyzed in the positive-ion mode using multiple reaction monitoring. Transitions of the precursors to the product ions were as follows: m/z 348.3›62.1 (for AEA detection) and 356.3›63.1 (for AEA-d8detection).
Western blots
Routine Western blotting procedures were used, as described previously (43). Briefly, samples of PVN and cortex were homogenized in radioimmunoprecipitation assay buffer containing a cocktail of protease inhibitors (Roche Diagnostics GmbH, Mannheim, Germany). Total protein concentration was determined using the bicinchoninic acid method with bovine serum albumin as a standard. Next, homogenates were reconstituted in Laemmli buffer, separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were immunoblotted with the primary antibodies of interest, followed by incubation with appropriate horseradish peroxidase-labeled secondary antibody. Equal protein concentration loading was controlled by Ponceau S staining. After adding a suitable substrate for horseradish peroxidase (Thermo Scientific, Rockford, IL, USA) protein bands were quantified densitometrically using a ChemiDoc visualization system EQ (Bio-Rad, Warsaw, Poland). The protein expression was standardized to β-tubulin.
Chemicals
Drugs used for the whole animal experiments were AM251, AM6545 (Sigma-Aldrich, St. Louis, MO, USA), CP55940, cremophor E1, R-(-)-methanandamide (Tocris Cookson, Bristol, UK), cyclodextrin, ruthenium red, kainic acid, urethane (Sigma, Munchen, Germany), SR144528 (Cayman Chemicals, Ann Arbor, MI, USA), pentobarbitone sodium (Biowet, Pulawy, Poland). Drugs were dissolved in saline with the following exceptions: AM251 was dissolved in a mixture of ethanol, Cremophor El, dimethyl sulphoxide (DMSO) and saline (1:1:1:9.5); methanandamide was purchased from Tocris Cookson as a 10 mg/ml emulsion in soya water (1:4); AM6545 was dissolved in DMSO using gentle heating before being diluted with Tween 80 and saline (4% DMSO; 1% Tween 80; 95% saline) for i.p. injections; CP55940 was dissolved in a 19% w/v solution of cyclodextrin. A stock solution of SR144528 was prepared in a mixture of DMSO, ethanol, and Cremophor El (2:1:1) and further diluted (1:10) in isotonic saline immediately before the experiment. The antibodies used in the Western blots were purchased from Cayman (anti-FAAH antibody, 1:200 dilution, cat. no. 101600), Novus Biological (anti-β-tubulin, 1:1000 dilution, cat. no. NB600-936) and Santa Cruz Biotechnology (anti-rabbit IgG horseradish peroxidase, 1:3000 dilution, cat. no. Sc-2004). The AEA and octadeuterated anandamide (AEA-d8) standards needed for the UPLC-MS/MS determinations were purchased from Cayman Chemical (Ann Arbor, MI, USA), whereas acetonitrile and ethanol were purchased from Sigma Chemical (St. Louis, MO, USA). All the stock solutions of standards were prepared in ethanol and stored at –80°C. Further dilutions were prepared using acetonitrile.
Data analysis
Results are given as means ± S.E.M. (n, number of rats). To quantify the effects of antagonists on the MethAEA- and CP55940-induced changes in cardiovascular parameters, S1 and S2 values were calculated as percent of the basal MBP, SBP, DBP and HR immediately before injection of the particular agonist. For comparison of the mean values, the t-test for paired and unpaired data was used, as appropriate.
RESULTS
General
Basal SBP, DBP, MBP and HR measured immediately before the administration of the agonist (S1 and S2) into the PVN are given in Table 1. The basal values of the four cardiovascular parameters determined before S1 and S2 did not differ, confirming that the cardiovascular effects induced by agonists during S1 ceased before S2. Basal values were not altered by the CB1 receptor antagonist AM251 (given i.v. or into the PVN), the peripherally restricted CB1 receptor antagonist AM6545 (i.p.), the CB2 receptor antagonist SR144528 (i.v.) and/or the TRPV1 receptor antagonist ruthenium red (i.v.) (Table 1). In the experiments related to Fig. 6 (see later), basal DBP and HR before PVN lesion were 72 ± 9 mmHg and 355 ± 19 beats/min (n = 4). Bilateral microinjection of 2 nmol kainic acid into the PVN resulted in immediate and marked increases in the baseline BP and HR (maximally by about 56 and 34% of basal values, respectively; P < 0.001), which gradually recovered within 40 – 60 min and 90 min, respectively; immediately before injection of CP55940, basal DBP and HR were 76 ± 9 mmHg and 378 ± 20 beats/min (n = 4), respectively. The above values of basal cardiovascular parameters were not different from the respective values obtained in control groups which have received the solvent for kainic acid (data not shown).
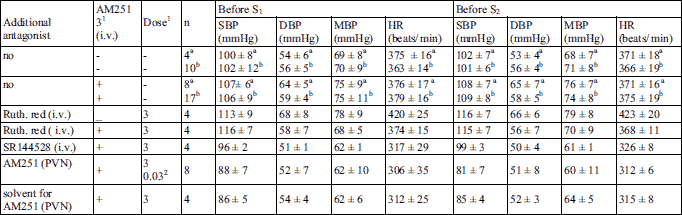
Cardiovascular effects of methanandamide given into the paraventricular nucleus of the hypothalamus
As shown in Fig. 1, the first microinjection (S1) of MethAEA (0.01 µmol/rat) into the PVN decreased SBP, DBP, MBP and HR by 15, 24, 15 and 4% of basal values (i.e. by 17 ± 4, 15 ± 3, 11 ± 2 mmHg and 13 ± 3 beats/min; n = 4), respectively. The second administration of the agonist (S2) into the PVN of the same rat induced comparable decreases. On the contrary, the cannabinoid CB1 antagonist AM251 3 µmol/kg given i.v. 5 min before S2 reversed the depressor and bradycardic effects of MethAEA; thus, SBP, DBP, MBP and HR were higher by 11, 15, 12 and 6% than the respective basal values. In contrast to AM251, i.v. injection of ruthenium red 3 µmol/kg 5 min before S2 did not modify the depressant effects of MethAEA on BP and HR. Both MethAEA-induced decreases and increases in cardiovascular parameters lasted for about 90 s.
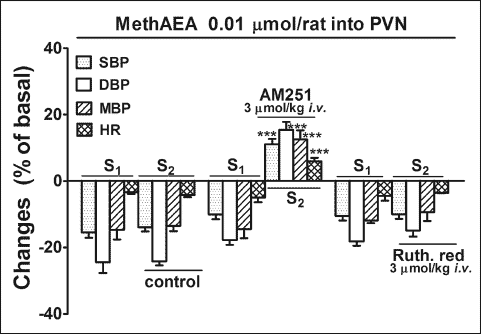 |
Fig. 1. Influence of methanandamide (MethAEA) microinjected into the PVN on systolic, diastolic and mean blood pressure (SDB, DBP, MBP) and heart rate (HR) and its interaction with AM251 or ruthenium red (Ruth. red) in urethane-anaesthetized rats. MethAEA was administered twice (S1 and S2, 20 min apart). AM251 or Ruth. red was given intravenously (i.v.) 5 min before S2. Results are calculated as percent of basal values determined immediately before S1 and S2. Means ± S.E.M. of four rats. ***P < 0.001 compared to the corresponding S1. All percentage changes obtained in S1 and S2 are significantly different with P < 0.05 compared to the respective basal values immediately before S1 and S2. |
Cardiovascular effects of CP55940 given into the paraventricular nucleus of the hypothalamus
The first microinjection of CP55940 (0.1 nmol/rat) into the PVN decreased DBP by about 25% and MBP and SBP by about 15% of basal values; the hypotensive effect lasted for 118 ± 8 s (n =4). A marginal bradycardia also occurred (Fig. 2). Almost identical changes in BP and HR were observed under control conditions during S2. Similarly to MethAEA, entirely different responses to CP55940 were observed 5 min after the i.v. administration of AM251. Thus, under CB1 receptor blockade, administration of CP55940 into the PVN increased SBP, DBP, MBP and HR by 20, 28, 24 and 5% of the basal value, respectively.
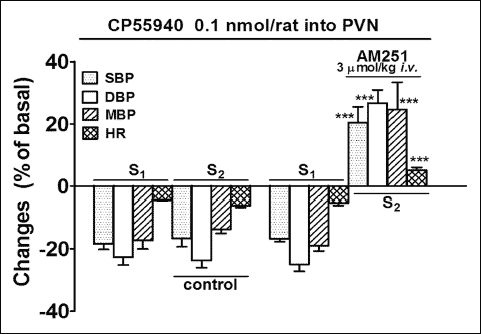 |
Fig. 2. Influence of CP55940 microinjected into the PVN on systolic, diastolic and mean blood pressure (SDB, DBP, MBP) and heart rate (HR) and its interaction with AM251 in urethane-anaesthetized rats. CP55940 was administered twice (S1 and S2, 20 min apart). AM251 was given intravenously (i.v.) 5 min before S2. Results are calculated as percent of basal values determined immediately before S1 and S2. Means ± S.E.M. of 25 (control) and 4 rats (AM251). ***P < 0.001 compared to the corresponding S1. All percentage changes obtained in S1 and S2 are significantly different with P < 0.05 compared to the respective basal values immediately before S1 and S2. |
Since the depressor and pressor effects of MethAEA and CP55940 were highest in the case of DBP, we have concentrated on the changes in DBP and HR in all further experiments. Fig. 3 shows the dose-response curves for CP55940 (0.01 – 1 nmol/animal). Most of its effects (determined before and after i.v. administration of AM251, respectively) were dose-dependent although its depressor effect was not. The maximal pressor response (about 30% of the basal value) was obtained for 0.1 nmol per animal. It lasted for 87 ± 9 s (n = 4) and was accompanied by an increase in HR by about 5% of the basal value. Thus, this dose was chosen for the subsequent studies.
Influence of CB1, CB2 and TRPV1 receptor antagonists on the pressor response to CP55940 given into the paraventricular nucleus of the hypothalamus
In the experiments of this section, the pressor effect of CP55940 microinjected into the PVN at a dose of 0.1 nmol/animal was examined in the presence of AM251 (3 µmol/kg; i.v.) given 5 min before S2.
The potential involvement of central CB1 receptors in the pressor effect of CP55940 was examined in two series of experiments. Firstly, we microinjected AM251 (0.03 µmol/rat) into the PVN during S2 5 min after its i.v. application (Fig. 4A). Secondly, we administered the peripherally restricted cannabinoid CB1 receptor antagonist AM6545 (15 µmol/kg, i.p.) 90 min before S1 (Fig. 4B). (As an exception, AM251 was not given i.v. and CP55940 was microinjected only once in the latter experiments). We found that the additional application of AM251 into the PVN reversed the pressor response to CP55940 microinjected into the PVN into a depressor one and reduced its tachycardic effect by about one third (P < 0.01). However, the CP55940-induced decrease in DBP was by about 40% lower in the presence of AM251 (S2) injected into the PVN than in its absence (S1; P < 0.05). On the contrary, after blockade of peripheral CB1 receptors by AM6545, microinjection of CP55940 into the PVN increased DBP and HR by 43 and 7% of the respective basal values (Fig. 4B); the duration of the pressor effect was 356 ± 24 s (n = 4). Importantly, the pressor effect of the same dose of CP55940 lasted four times longer (P < 0.001) and was stronger by 60% (P < 0.05) in the presence of AM6545 (Fig. 4B) than after AM251 (Fig. 2).
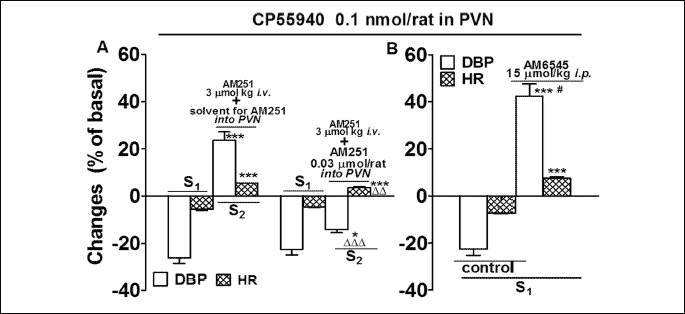
As shown in Fig. 5, the non-selective TRPV1 receptor antagonist ruthenium red (3 µmol/kg; i.v.) and the CB2 receptor antagonist SR144528 (3 µmol/kg; i.v.) given in addition to AM251 (3 µmol/kg; i.v.) 5 min before S2 did not affect the increases in DBP and HR induced by microinjection of CP55940 into the PVN.
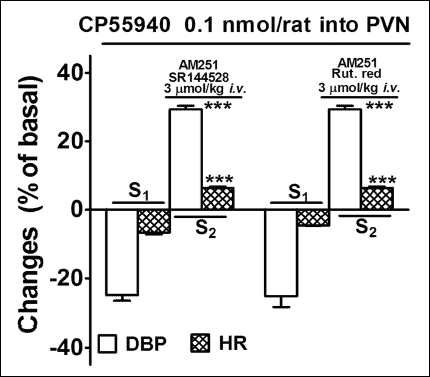 |
Fig. 5. Influence of SR144528 and ruthenium red (Ruth. red) on the increase in diastolic blood pressure (DBP) and heart rate (HR) induced by CP55940 microinjected into the PVN in urethane-anaesthetized rats. CP55940 was administered twice (S1 and S2, 20 min apart). SR144528 or Ruth. red was administered intravenously (i.v.) together with AM251, 5 min before S2. Results are calculated as percent of basal values determined immediately before S1 and S2. Means ± S.E.M. of 4 rats. ***P < 0.001 compared to the corresponding S1. All percentage changes obtained in S1 and S2 are significantly different with P < 0.05 compared to the respective basal values immediately before S1 and S2. |
Effects of peripheral CB1 receptor blockade and chemical lesion of the paraventricular nucleus of the hypothalamus on the cardiovascular effects of CP55940 given i.v.
Intravenous administration of CP55940 (1 – 100 nmol/kg) induced decreases in all four cardiovascular parameters (Fig. 6). The highest dose reduced DBP, SBP, MBP and HR by 33, 27, 25 and 16% of basal values, respectively. Blockade of the peripheral CB1 receptors by AM6545 given i.p. 90 min before the first dose of the agonist reversed the depressant effects of CP55940 into stimulatory ones. Thus, in the presence of this antagonist, CP55940 dose-dependently increased the four cardiovascular parameters; at 100 nmol/kg, the increases in BP and HR were by about 30 and 10% of the basal values, respectively. The depressor effects of CP55940 were much shorter than its pressor effects. Thus, the fall in DBP induced by the highest dose of CP55940 100 nmol/kg lasted for 28 ± 5 min (n = 4). On the contrary, 30 min after the administration of the same dose of CP55940, DBP was still increased by about 15% of the basal value.
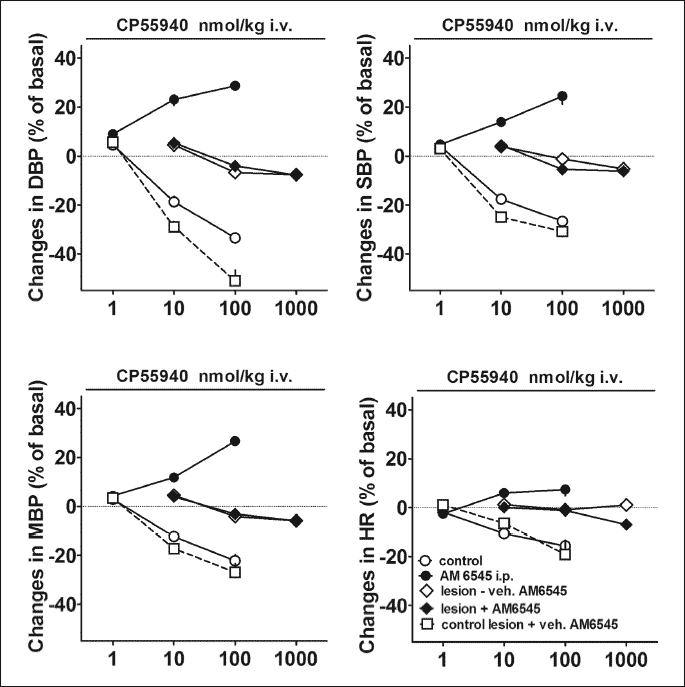
As shown in Fig. 6, bilateral chemical lesion of the PVN induced by injection of kainic acid abolished all cardiovascular effects of CP55940 given i.v. Thus, neither depressor nor pressor responses (in the presence of AM6545) to CP55940 were observed (even when the latter was applied at a tenfold higher dose than before).
The solvents for kainic acid (given into the PVN) or for AM6545 (given i.p.) failed to modify the respective cardiovascular effects of CP55940 (Fig. 6).
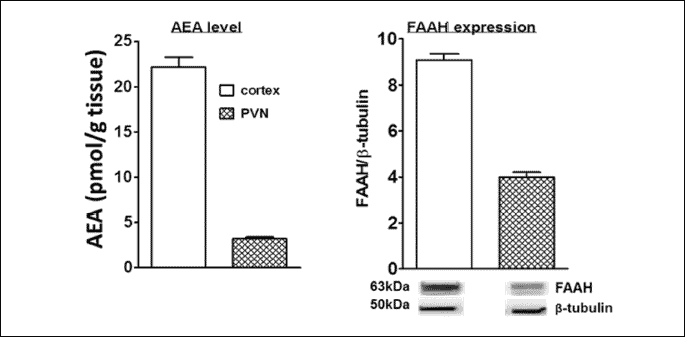
Anandamide levels and expression of fatty acid amide hydrolase protein in the rat cerebral cortex and paraventricular nucleus of the hypothalamus
The AEA level in the rat cerebral cortex and PVN was quantified using UPLC-MS/MS and amounted to 22 ± 1 and 3 ± 0.2 pmol/g tissue (n = 8), respectively (Fig. 7). The expression of fatty acid amide hydrolase (FAAH) (an enzyme responsible for AEA degradation) was analyzed by Western blotting (Fig. 7). Western blot analysis showed a single immunoreactive band of the molecular size expected for FAAH (63 kDa) in cortex and PVN.
DISCUSSION
We examined the potential involvement of the PVN, a key area integrating sympathetic outflow (28), in the cannabinoid-induced changes of cardiovascular parameters in anaesthetized rats. For this purpose, we microinjected directly into the PVN (a) MethAEA, which given i.v. mimics the well-known triphasic changes in cardiovascular parameters induced by AEA (i.v.) (7), and (b) CP55940, a synthetic agonist of cannabinoid receptors (44), which decreases BP after its i.v. injection only (5). We preferred urethane over pentobarbitone anesthesia since pentobarbitone reduces the AEA-stimulated increase in BP (10) and under urethane anaesthesia the autonomic nervous system remains tonically active in the control of the cardiovascular parameters (45). Moreover, urethane (but not pentobarbitone) increases the resting sympathetic tone, since its i.v. administration enhances plasma adrenaline and noradrenaline concentrations (46, 47) and noradrenaline release from the PVN (47). With the exception of AM6545 (see later) all receptor ligands given i.v. in the present study penetrate the blood-brain barrier (48-50).
MethAEA given into the PVN decreased BP by 20% and HR by 5%. Our data with the TRPV1 receptor antagonist ruthenium red and the slow administration of all compounds into the PVN argue against the involvement of the TRPV1 receptor-mediated Bezold-Jarisch reflex, which is induced by rapid i.v. injection only (7). By contrast, the i.v. injection of the CB1 receptor antagonist AM251 not only reduced but even reversed the depressor effect of MethAEA into a pressor one. Similarly, we had previously shown for AEA that combined i.v. administration of AM251 and ruthenium red reversed its (i.c.v.) hypotensive response into a pressor effect (8). The pressor effect of MetAEA cannot be a reflex response to the hypotension during S1, because it was induced 20 min after S1. Moreover, we have excluded previously that the pressor effect of AEA is just a reflex response to a preceding hypotension since it was not modified by vagotomy (10).
In conscious rats (12) and rabbits (18), CB1 receptors are postulated to modulate the pressor effects of the cannabinoids CP55940,WIN55212-2 and HU-210. Thus, we decided to examine the cardiovascular effects of CP55940 given into the PVN. We chose CP55940 (a high-efficacy agonist of CB1 and CB2 receptors; (44)) for our studies since its central and peripheral cardiovascular effects have been described in detail (1). Similarly to MethAEA, CP55940 caused a dose-dependent fall in BP (which was strongest in the case of DBP; expressed in % of basal values) and a modest decrease in HR. We have concentrated on the changes in DBP which reflect changes in the peripheral resistance. Importantly, after AM251 (i.v.) a dose-dependent pressor effect and slight tachycardia occurred. These effects were not further modified by SR144528 suggesting that CP55940 does not act via CB2 receptors in our experimental model.
In previous studies, administration of AEA, WIN55212-2 or HU-210 i.c.v. (20) into the RVLM (21) or dPAG (22) of anaesthetized rats increased BP and/or renal sympathetic nerve activity in a manner sensitive to CB1 antagonists. By contrast, in our paper microinjection of MetAEA and CP55940 decreased BP. The above hypotensive effects of both agonists were reversed into pressor responses by the CNS-penetrating CB1 antagonist AM251 and/or the peripherally restricted CB1 antagonist AM6545. One might assume that CP55940 injected into the PVN produces a sympathetic activation accompanied by an increased formation of endocannabinoids or an increase in the constitutive activity of the CB1 receptors in the vascular sympathetic nerve endings. In the absence of an inverse CB1 agonist/antagonist the endocannabinoids and/or the high constitutive activity may override the central pressor effect of CP55940, thereby leading to hypotension.
The shift from the hypo- to a hypertensive response of the cannabinoids might, however, also be related to the fact that the final integration of the sympathetic outflow by the PVN results from the balance between stimulatory and inhibitory inputs depending on glutamate and GABA, respectively (28, 51). Presynaptic CB1 receptors inhibit the release of either transmitter (52). In the conscious rat with a normal sympathetic tone, CB1 receptor-related inhibition of GABA release leads to sympathoexcitation and a BP increase (19). However, under urethane anaesthesia a high resting sympathetic tone occurs (46) leading to a higher inhibitory effect of cannabinoids on glutamate than on GABA release associated with a hypotensive effect. It is an intriguing idea that blockade of peripheral CB1 receptors via an afferent reflex leads to a shift from the predominance of CP55940 at CB1 receptors on glutamatergic to those at GABAergic neurons leading to hypertension. Admittedly, the question whether the cardiovascular effects of the two cannabinoids are related to the latter phenomena or to a more peripheral mechanism as outlined in the previous paragraph cannot be decided so far.
In the present paper, we have concentrated on the hypertensive effects of cannabinoids. The increase in BP induced by CP55940 was further studied in experiments in which we microinjected CP55940 into the PVN together with AM251 and 5 min after AM251 (i.v.). Combined topical plus systemic administration of AM251 counteracted the pressor effect of CP55940 suggesting that CB1 receptors in the PVN are implicated in its pressor effect. These CB1 receptors may be the target of endogenously formed endocannabinoids, as postulated by Gyombolai et al. (33). The occurrence of one of the endocannabinoids, i.e. AEA, in the PVN has been proven by us with the UPLC-MS/MS method. The enzyme involved in the degradation of AEA, fatty acid amide hydrolase, has been identified as well, using the Western blot technique. The fact that CP55940, after topical and i.v. administration of AM251, even led to a depressor response (which was, however, less pronounced than that obtained in the absence of AM251) points to an additional, CB1 receptor-independent hypotensive effect of CP55940.
In further experiments, CP55940 administered via the i.v. route induced the well-known, dose-dependent fall in all cardiovascular parameters (5) without any pressor effect but, after AM6545 i.p., elicited a very strong and prolonged increase in BP. These results confirm that peripheral CB1 receptors are responsible for prolonged hypotension (1). Moreover, bilateral PVN chemical lesion with kainic acid abolished the pressor and depressor responses to CP55940 both in the absence or presence of AM6545. These results again suggest the important role of the PVN in the depressor and pressor effects of cannabinoids. But why did CP55940 (i.v.) not decrease BP in the PVN-lesioned rats? One might have expected a depressor effect since CP55940 should activate the CB1 receptors on the sympathetic nerve endings in the cardiovascular system in this experimental series; moreover, basal cardiovascular parameters had fully recovered after the lesion at the time point of CP55940 administration. The explanation may be that the PVN lesion has such a fundamental effect on the sympathetic outflow that its peripheral modulation via CB1 receptors is lost. Similarly, chemical PVN lesion completely abolished the increases in renal sympathetic activity and BP induced by the adipose afferent reflex (39).
Why did intravenous injection of AM251 block the depressor but not the pressor response to MethAEA and CP55940 given into the PVN? We suppose that AM251 (i.v.) could not reach the PVN in sufficient amounts. Studies with [123I]AM251 performed on baboons have demonstrated a poor brain penetration of this antagonist (48) attributed to its extremely high lipophilicity (cLogP 7.36) or to a tight, specific association to a plasma protein. Nonetheless, a partial inhibition of central CB1 receptors is suggested by the fact that the pressor response to CP55940 after i.v. AM251 was smaller and shorter than that after i.p. AM6545.
The depressor and pressor responses induced by PVN microinjection of MethAEA and CP55940 were accompanied by only slight bradycardia and tachycardia, respectively. Changes in HR were modified by various pharmacological tools employed in our study usually in the same way like the changes in BP and are probably mediated by the same mechanisms.
The PVN is a site in which signals associated with the body energy status determine the direction of the effects of endocannabinoids on food intake via CB1 receptors (hyper- or hypophagia) (29). Similarly, the sympathetic tone may determine the blood pressure response to cannabinoids via CB1 receptors in the PVN. Accordingly, in anaesthetized spontaneously hypertensive rats (SHR) with a higher sympathetic tone than in normotensive rats, a longer hypotensive effect of AEA or AM3506 (an inhibitor of FAAH) was observed (6, 53). A hypotensive response was also reported in acute hypertensive conscious rats (54) whereas an increase in BP occurred in normotensive rats that exhibit a lower sympathetic tone (12, 54).
In conclusion, our results show that in addition to its well-known hypotensive effect related to the stimulation of peripheral CB1 receptors, CP55940 injected into the PVN of urethane-anaesthetized rats can induce a depressor and pressor effect accompanied with a less marked bradycardia or tachycardia, respectively. The direction of the response is probably dependent on the sympathetic tone. Importantly, the pressor effect of cannabinoids is unmasked and strongly enhanced in the presence of peripheral CB1 receptor blockade. Consequently, one has to consider that peripherally restricted CB1 receptor antagonists, which may become new therapies for controlling obesity and related disorders (27, 37, 55), may increase blood pressure; particularly in patients with a metabolic syndrome, the latter effect may outweigh the beneficial effect on body weight.
Abbreviations: AEA, anandamide; AM251, N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; AM6545, 5-(4-(4-cyanobut-l-ynyl)phenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(l,l-dioxothiomorpholino)-lH-pyrazole-3-carboxamide; BP, blood pressure; CP55940, (-)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol; DBP, diastolic blood pressure; DMSO, dimethyl sulfoxide; dPAG, dorsal periaqueductal gray; FAAH, fatty acid amide hydrolase; HR, heart rate; MBP, mean blood pressure; MethAEA, methanandamide; PVN, paraventricular nucleus of the hypothalamus; RVLM, rostral ventrolateral medulla; SBP, systolic blood pressure; SR144528, N-[(1S)-endo-1,3,3-trimethylbicyclo[2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide; Δ9-THC, Δ9-tetrahydrocannabinol; UPLC-MS/MS, ultra-high performance liquid chromatography-tandem mass spectrometry
Acknowledgements: The work of B.M. and E.S. was supported by a grant from the Medical University of Bialystok (Poland; grant No. 113-13614F and 123-13882F) and by the Deutsche Forschungsgemeinschaft (SCHL 266/9-2), respectively.
Conflict of interests: None declared.
REFERENCES
- Malinowska B, Baranowska-Kuczko M, Schlicker E. Triphasic blood pressure responses to cannabinoids: do we understand the mechanism? Br J Pharmacol 2012; 165: 2073-2088.
- Penumarti A, Abdel-Rahman AA. The novel endocannabinoid receptor GPR18 is expressed in the rostral ventrolateral medulla and exerts tonic restraining influence on blood pressure. J Pharmacol Exp Ther 2014; 349: 29-38.
- Stanley C, O’Sullivan SE. Vascular targets for cannabinoids: animal and human studies. Br J Pharmacol 2014; 171: 1361-1378.
- Zubrzycki M, Liebold A, Janecka A, Zubrzycka M. A new face of endocannabinoids in pharmacotherapy. Part I: Protective role of endocannabinoids in hypertension and myocardial infarction. J Physiol Pharmacol 2014; 65: 171-181.
- Lake KD, Campton DR, Varga K, Martin BR, Kunos G. Cannabinoid-induced hypotension and bradycardia in rats is mediated by CB1-like cannabinoid receptors. J Pharmacol Exp Ther 1997; 281: 1030-1037.
- Lake KD, Martin BR, Kunos G, Varga K. Cardiovascular effects of anandamide in anaesthetized and conscious normotensive and hypertensive rats. Hypertension 1997; 29: 1204-1210.
- Malinowska B, Kwolek G, Gothert M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn-Schmiedeberg’s Arch Pharmacol 2001; 364: 562-569.
- Malinowska B, Zakrzeska A, Kurz CM et al. E. Involvement of central β2-adrenergic, NMDA and thromboxane A2 receptors in the pressor effect of anandamide in rats. Naunyn-Schmiedeberg’s Arch Pharmacol 2010; 381: 349-360.
- Pacher P, Batkai S, Kunos G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J Physiol 2004; 558: 647-657.
- Kwolek G, Zakrzeska A, Schlicker E, Gothert M, Godlewski G, Malinowska B. Central and peripheral components of the pressor effect of anandamide in urethane-anaesthetized rats. Br J Pharmacol 2005; 145: 567-575.
- Zakrzeska A, Schlicker E, Baranowska M, Kozlowska H, Kwolek G, Malinowska B. A cannabinoid receptor, sensitive to O-1918, is involved in the delayed hypotension induced by anandamide in anaesthetized rats. Br J Pharmacol 2010; 160: 574-584.
- Gardiner SM, March JE, Kemp PA, Bennett T. Influence of the CB1 receptor antagonist, AM 251, on the regional haemodynamic effects of WIN-55212-2 or HU 210 in conscious rats. Br J Pharmacol 2002; 136: 581-587.
- Gardiner SM, March JE, Kemp PA, Bennett T. Factors influencing the regional haemodynamic responses to methanandamide and anandamide in conscious rats. Br J Pharmacol 2009; 158: 1143-1152.
- O’Sullivan SE, Randall MD, Gardiner SM. The in vitro and in vivo cardiovascular effects of Δ9-tetrahydrocannabinol in rats made hypertensive by chronic inhibition of nitric-oxide synthase. J Pharmacol Exp Ther 2007; 321: 663-672.
- Malinowska B, Godlewski G, Bucher B, Schlicker E. Cannabinoid CB1 receptor-mediated inhibition of the neurogenic vasopressor response in the pithed rat. Naunyn-Schmiedeberg’s Arch Pharmacol 1997; 356: 197-202.
- Niederhoffer N, Schmid K, Szabo B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn-Schmiedeberg’s Arch Pharmacol 2003; 367: 434-443.
- Malinowska B, Piszcz J, Koneczny B, Hryniewicz A, Schlicker E. Modulation of the cardiac autonomic transmission of pithed rats by presynaptic opioid OP4 and cannabinoid CB1 receptors. Naunyn-Schmiedeberg’s Arch Pharmacol 2001; 364: 233-241.
- Niederhoffer N, Szabo B. Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J Pharmacol Exp Ther 2000; 294: 707-713.
- Ibrahim BM, Abdel-Rahman AA. Role of brainstem GABAergic signaling in central cannabinoid receptor evoked sympathoexcitation and pressor responses in conscious rats. Brain Res 2011; 1414: 1-9.
- Pfitzer T, Niederhoffer N, Szabo B. Central effects of the cannabinoid receptor agonist WIN55212-2 on respiratory and cardiovascular regulation in anaesthetized rats. Br J Pharmacol 2004; 142: 943-952.
- Padley JR, Li Q, Pilowsky PM, Goodchild AK. Cannabinoid receptor activation in the rostral ventrolateral medulla oblongata evokes cardiorespiratory effects in anaesthetized rats. Br J Pharmacol 2003; 140: 384-394.
- Dean C. Endocannabinoid modulation of sympathetic and cardiovascular responses to acute stress in the periaqueductal gray of the rat. Am J Physiol Regul Integr Comp Physiol 2011; 300: R771-R779.
- Varga K, Lake K, Huangfu D, Guyenet PG, Kunos G. Mechanism of hypotensive action of anandamide in anaesthetized rats. Hypertension 1996; 28: 682-686.
- Krowicki ZK. Involvement of hindbrain and peripheral prostanoids in gastric motor and cardiovascular responses to delta-9-tetrahydrocannabinol in the rat. J Physiol Pharmacol 2012; 63: 581-588.
- Villanueva A, Yilmaz SM, Millington WR, et al. Central cannabinoid 1 receptor antagonist administration prevents endotoxic hypotension affecting noradrenaline release in the preoptic anterior hypothalamic area. Shock 2009; 32: 614-620.
- Batkai S, Pacher P, Osei-Hyiaman D, Radavea S, et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation 2004; 110: 1996-2002.
- Silvani A, Berteotti C, Bastianini S, et al. Cardiorespiratory anomalies in mice lacking CB1 cannabinoid receptors. PLoS One 2014; 20: e100536.
- Pyner S. The paraventricular nucleus and heart failure. Exp Physiol 2014; 99: 332-339.
- Bellocchio L, Lafenetre P, Cannich A, et al. Bimodal control of stimulated food intake by the endocannabinoid system. Nature Neurosci 2010; 13: 281-283.
- Wierucka-Rybak M, Wolak M, Bojanowska E. The effects of leptin in combination with a cannabinoid receptor 1 antagonist, AM251, or cannabidiol on food intake and body weight in rats fed a high-fat or a free-choice high sugar diet. J Physiol Pharmacol 2014; 65: 487-496.
- Wamsteeker JI, Kuzmiski JB, Bains JS. Repeated stress impairs endocannabinoid signaling in the paraventricular nucleus of the hypothalamus. J Neurosci 2010; 30: 11188-11196.
- Castelli MP, Piras AP, Melis T, et al. Cannabinoid CB1 receptors in the paraventricular nucleus and central control of penile erection: immunocytochemistry, autoradiography and behavioral studies. Neuroscience 2007; 147: 197-206.
- Gyombolai P, Pap D, Turu G, Catt KJ, Bagdy G, Hunyady L. Regulation of endocannabinoid release by G proteins: a paracrine mechanism of G protein-coupled receptor action. Mol Cell Endocrinol 2012; 353: 29-36.
- Paxinos G, Watson C. The rat Brain in Stereotaxic Coordinates. Elsevier Academic Press, Boston. 2007.
- Miller CC, Murray TF, Freeman KG, Edwards GL. Cannabinoid agonist, CP 55,940, facilitates intake of palatable foods when injected into the hindbrain. Physiol Behav 2004; 80: 611-616.
- Rudz R, Schlicker E, Baranowska U, Marciniak J, Karabowicz P, Malinowska B. Acute myocardial infarction inhibits the neurogenic tachycardic and vasopressor response in rats via presynaptic cannabinoid type 1 receptor. J Pharmacol Exp Ther 2012; 343: 198-205.
- Cluny NL, Vemuri VK, Chambers AP, et al. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol 2010; 161: 629-642.
- Chapman CD, Dono LM, French MC, Weinberg ZY, Schuette LM, Currie PJ. Paraventricular nucleus anandamide signaling alters eating and substrate oxidation. Neuroreport. 2012; 23: 425-429.
- Shi Z, Chen WW, Xiong XQ, et al. Sympathetic activation by chemical stimulation of white adipose tissues in rats. J Appl Physiol (1985) 2012; 112: 1008-1014.
- Du D, Jiang M, Liu M, et al. Microglial P2X7 receptor in the hypothalamic paraventricular nuclei contributes to sympathoexcitatory responses in acute myocardial infarction rat. Neurosci Lett 2015; 587: 22-28.
- Lam PM, Marczylo TH, El-Talatini M, et al. Ultra performance liquid chromatography tandem mass spectrometry method for the measurement of anandamide in human plasma. Anal Biochem 2008; 380: 195-201.
- Marczylo TH, Lam PM, Amoako AA, Konje JC. Anandamide levels in human female reproductive tissues: solid-phase extraction and measurement by ultraperformance liquid chromatography tandem mass spectrometry. Anal Biochem 2010; 400: 155-162.
- Chabowski A, Zendzian-Piotrowska M, Konstantynowicz K, et al. Fatty acid transporters involved in the palmitate and oleate induced insulin resistance in primary rat hepatocytes. Acta Physiol (Oxf) 2013; 207: 346-357.
- Pertwee RG, Howlett AC, Abood ME, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 2010; 62: 588-631.
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 2: Cardiovascular system. Experientia 1986; 42: 292-297.
- Carruba MO, Bondiolotti G, Picotti GB, Catteruccia N, Da Prada M. Effects of diethyl ether, halothane, ketamine and urethane on sympathetic activity in the rat. Eur J Pharmacol 1987; 134: 15-24.
- Shimokawa A, Jin QH, Ishizuka Y, Kunitake T, Takasaki M, Kannan H. Effects of anesthetics on norepinephrine release in the hypothalamic paraventricular nucleus region of awake rats. Neurosci Lett 1998; 244: 21-24.
- Gatley SJ, Lan R, Volkow ND, et al. Imaging the brain marijuana receptor: development of a radioligand that binds to cannabinoid CB1 receptors in vivo. J Neurochem 1998; 70: 417-423.
- Petitet F, Jeantaud B, Bertrand P, Imperato A. Cannabinoid penetration into mouse brain as determined by ex vivo binding. Eur J Pharmacol 1999; 374: 417-421.
- Feria-Velasco A, Morales-Villagran A, Tapia-Arizmendi G, Arauz-Contreras J, Espinosa de los Monteros A. Effects of ruthenium red intraperitoneally injected to adult rats, on the uptake and release of neurotransmitters from brain synaptosomes. Arch Invest Med (Mex) 1990; 21: 45-50.
- Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol 2006; 291: H2847-H2856.
- Szabo B, Schlicker E. Effects of cannabinoids on neurotransmission. Handb Exp Pharmacol 2005; 168: 327-365.
- Godlewski G, Alapafuja SO, Batkai S, et al. Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem Biol 2010; 17: 1256-1266.
- Ho WS, Gardiner SM. Acute hypertension reveals depressor and vasodilator effects of cannabinoids in conscious rats. Br J Pharmacol 2009; 156: 94-104.
- Chorvat RJ. Peripherally restricted CB1 receptor blockers. Bioorg Med Chem Lett 2013; 23: 4751-4760.
A c c e p t e d : March 9, 2015