PHYSIOLOGICAL AND ENVIRONMENTAL FACTORS ASSOCIATED WITH CENTRAL FAT DISTRIBUTION IN PUBERTAL GIRLS
INTRODUCTION
A distribution of adipose tissue is one of the phenomena that change during the maturation process: with the age the amount of subcutaneous fat decreases in the extremities region while it relatively increases in the region of trunk. The process begins already at the preschool period and significantly intensifies during pubescence and its rate is sexually diverse. A more dynamic movement of subcutaneous adipose tissue towards trunk region takes place in boys and they are characterised by a more central type of body fat distribution in comparison to a peripheral type in girls (1, 2). Due to the fact that the excessive central distribution of adipose tissue is connected with several health consequences, also in children, lately more attention has been focused on anthropometric indices which easily allow to assess abdominal fat distribution in population studies. Children abdominal skin-fold thickness correlates well with visceral adipose tissue as measured by a computerized axial tomography scan and magnetic resonance imaging (3). However, skin-fold thickness varies with age, sex and race and the equations relating skin-fold thickness at several sites to total body fat need to be validated for each population. There has been a recent interest in the use of the waist-to-height ratio (WHtR) for identifying excessive central adiposity in children and adolescents (4-6). Waist circumference (WC) and waist-to-height ratio (WHtR) have been shown to correlate with amount of abdominal fat, as well as cardiovascular and metabolic risk factors (4). WHtR is a relatively new index and some studies in children suggest that WHtR provides a superior indicator of adverse metabolic profiles compared with body mass index (BMI) (5, 6), whereas some others indicate a similar correlation between BMI and WHtR with cardiometabolic risk factors (7, 8).
The use of genetic material, being the most important factor determining growth and pubescence in a child, depends on a few cooperating elements. These can be internal factors with a leading role of the neurohormonal system as well as the external ones - environmental, socio-economic conditions, lifestyle, nutrition or physical activity. The factors also participate in the process of energy accumulation and expenditure. Recently, scientists have focused on a role of ghrelin and leptin - the key hormones taking part in a long-term control of taking food and body mass regulation. Leptin, due to its activity relying on decreasing appetite, is called the satiety hormone. It is synthesized and secreted mainly by white adipose tissue cells and, to a little degree, by skeletal muscles, hypophysis, brain, stomach, placenta, mammary gland and heart. It makes a negative feedback cell between adipose tissue and centres located in arcuate, ventromedial, lateral, paraventicular and supraoptic nuclei of hypothalamus (9). The main effect of leptin activity is retarding the synthesis and secretion of neuropeptide Y (NPY) strongly stimulating food intake in arcuate nucleus. Inhibiting secretion of NPY increases energy expenditure through a growth of thermogenesis, activates lipolysis and decelerates lipogenesis (10). Besides regulation through NPY, leptin slows down the activity of the cannabinoid system in hypothalamus (11) connected with feeling satisfied after consuming sweets. Another substance - ghrelin is engaged in fast-acting regulation of energy homeostasis by applying a stimulating influence on hunger centre (12). It is secreted in a pulsatile way by mucosa cells of the stomach fundus (12) and further part of the alimentary track (13). From among all peptides that have been identified so far, ghrelin indicated the strongest orexigenic action, therefore it increases food intake and fat accumulation in humans (14). Ghrelin stimulates food intake through activation of NPY neurones (neuropeptide Y)/AgRP (agouti related peptide) in arcuate nucleus and neurones in the lateral side of hypothalamus producing orexins A and B (neuropeptides stimulating appetite) (15). It also strengthens feeling of satisfaction related to food intake (16). Ghrelin is a protein that increases secretion of growth hormone in the brain hypophysis, too (12).
The aim of the work was to determine a degree of explanation of the variation of a central type of body fat distribution described by the waist-to-height ratio (WHtR) and waist circumference (WC) as well as the body mass index (BMI) by both environmental and biological factors, including hormonal ones, and also to define factors which are significantly connected with a risk of abdominal obesity in pubertal girls from Cracow.
MATERIAL AND METHODS
Subjects
The study material includes a cross-sectional sample of 297 girls aged 9 – 16 years (12.92 ± 2.08), examined in two types of schools in the city of Cracow, Poland: sport and regular ones. The girls from sport schools (n = 194) participated in regular swimming trainings for at least 10 hours weekly. Whereas, the girls from regular schools (n = 103) took part in physical education classes for 4 hours a week. According to the Polish law, children’s parents take a decision of sending them to a sport school but the results of fitness tests verifying basic motor abilities like strength, agility, flexibility and speed decide whether a child is enrolled.
The calendar age of the subjects, calculated as a difference between the date of the examination and the birth date and expressed as a decimal fraction, was a basis for classifying them into age groups, e.g., the ones aged 8.50 – 9.49 were the group of 9-year-old children. Children’s parents’ or guardians’ approvals as well as a permission from the Bioethical Commission were obtained to conduct the research.
The study was a part of the project no. 214/KF/2006 Suder and Plonka, University of Physical Education, Cracow: ‘Concentration of ghrelin, leptin and growth hormone (GH) in prepubertal girls vs. distribution of adipose tissue in the aspect of differentiated physical activity (longitudinal studies)’.
Anthropometry
Anthropometric measurements were taken by anthropologists in the morning in separate rooms. For the purpose of this study body height (Ht), body weight (W) and waist circumference (WC) were used. Ht was measured in the examined without shoes, in standing position to the nearest 0.1 cm, with the head in the Frankfurt plane, with the use of an anthropometer. Weight was obtained in the standing position with a standardized medical scale with an accuracy of 100 g. WC was measured to the nearest 0.1 cm by using an anthropometric tape between the lower edge of costal arch and the upper edge of iliac crest with the subjects in standing position, recorded at the end of a gentle expiration. The body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Waist-to-height ratio (WHtR) was calculated by dividing waist circumference (in cm) by height (in cm). Abdominal obesity was determined based on the WHtR cut-off points ≥ 0.50 (17). Fat mass (FM), percentage of body fat (% FAT) and fat-free mass (FFM) were estimated with bioelectrical impedance analysis (BIA), using a Body Composition Analyzer TBF 300 (Tanita Corp., Tokyo, Japan). The BIA test was carried out in the morning (fasting) after the subjects had emptied their bladders, according to procedures provided by the manufacturer. Prediction equations suggested by the manufacturer take into account, among others, sex, age, body weight and height of the examined patients (18).
In each girl the phase of breast development was assessed during a medical examination performed by a medical doctor in a school surgery with the use of the Tanner stage (19). The stage is a commonly applied measure of sexual maturation progression based on the level of secondary sex characters development. In girls the development level of breast and pubic hair is assessed. However, in the presented cross-sectional tests only the level of breast development was determined. Based on the phases the girls were qualified to one of four separate groups: due to a low number of patients qualified to the fifth group, the girls were included into the fourth one.
Biochemical (hormonal) analyses
Blood samples were collected after overnight fast, between 8 and 9 a.m. The concentration of leptin and ghrelin in blood serum was determined at the Isotope Laboratory (Department of Physiology, Jagiellonian University Medical College, Cracow) using radioimmunoassay method for human leptin (h-Leptin RIA kit, Linco Research, Missouri, USA) and ghrelin (h-Ghrelin RIA kit, Peninsula Laboratories Inc., Division Bachem, CA, USA).
The questionnaire study
The girls’ lifestyles and their families’ socio-economic status were investigated through survey questionnaires. The questionnaire was used to assess parents’ height and weight, parents’ education level, having siblings, regularity of children’s breakfasts and also a number of hours of physical activity spent by children during school classes (sport or regular ones). The parents’ body mass index was determined based on their body weight and height data obtained from questionnaires and parental obesity was defined as BMI exceeding 30. Parents’ educational level was defined as low (ground, basic vocational and secondary comprehensive) and high (incomplete university or university). Presence of siblings was categorized dichotomously: yes/no. Girls attending sport schools had regular swimming training on average 6.93 ± 4.98 month/year, 5 ± 1.86 days/week and 2.20 ± 1.07 hours/day and were categorized to the group of high physical activity. The examined attending a regular school who were physically active on average for 6.94 ± 4.15 month/year, 1.69 ± 1.09 days/week and 1.11 ± 0.46 hours/day were categorized to the group of low physical activity.
Statistical analysis
A relevant database and all statistical analyses were made using the Statistica 10.0 software package. During the first stage of working out the data, the basic statistical characteristics of somatic features were assessed in girls included in the study according to the Tanner stage. The Kruskal-Wallis test was used to specify differences between the Tanner groups. Differentiation in anthropometric features and indices was also estimated in groups of girls distinguished because of the school type. The significance of differences between the two groups was checked with the nonparametric Mann-Whitney test. Due to skewed distribution of features, established with Shapiro-Wilk test (non-included data) measurements were transformed into logarithmic scale (log). Values of somatic traits were standardized which enabled elimination of titre and an influence of the features size.
A stepwise descending multiple regression method was applied to evaluate a degree of waist circumference (WC), waist-to-height ratio (WHtR) and body mass index (BMI) variation explanation. Consecutively, a variable with the highest P >a was eliminated and, following each elimination, the model was subjected to new assessment. Elimination of variables was carried out until all structural parameters left in the model were statistically significant. Three separate full models were created with WC, WHtR and BMI as dependent variables. Standardized fractional regression coefficients showed the influence the particular independent variables (biological and environmental ones) had on a dependant variable (WC, WHtR, BMI) after eliminating influences of the remaining variables included in the analysis.
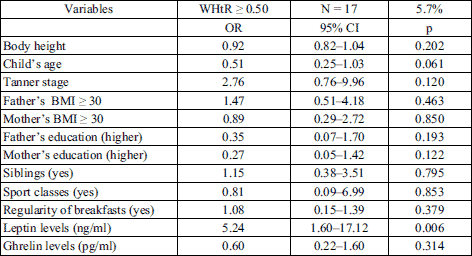
In the last stage, the values of WHtR ≥ 0.50 were applied as the cut-off points that distinguish between normal and abdominally obese girls (n = 17; 5.7%). A logistic regression analysis was conducted to indicate factors connected with a risk of abdominal obesity in the examined girls. Odds ratio (OR) and 95% confidence interval (CI) were calculated. Significance of the differences was set of the level of P < 0.05.
RESULTS
A comparative analysis conducted in groups distinguished due to a degree of pubescence progression (the Tanner stage) indicated a growth of value in indices describing body fatness in the examined girls, including BMI, with an increase of a degree of pubescence (Table 1). Parallel, changes in the concentration level of leptin in blood serum were taking place. Concentration of ghrelin in the examined girls did not indicate statistically significant changes between the girls groups according to the Tanner stage. Mean values of the WHtR index presented high stability and, unlike WC, no clear tendencies of their changes together with a degree of pubescence progression were observed.
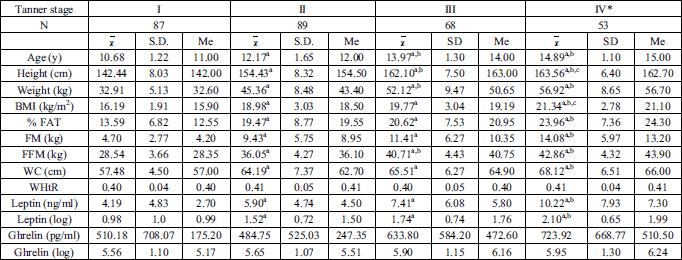
Table 2 presents basic statistical characteristics of the girls in groups distinguished due to the school type. The girls from sport classes and regular ones were in a similar age. Significant statistical differences in mean values of body height and weight between the girls from the two types of schools were not confirmed. The girls differed statistically in mean values of WC, WHtR and mean level of leptin and ghrelin in blood serum.
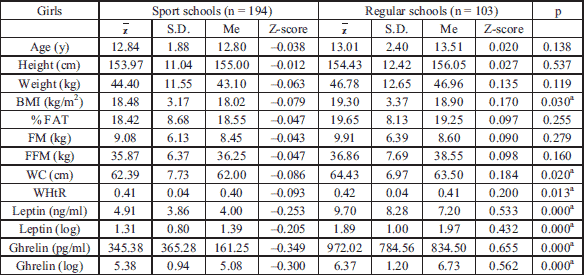
Table 3 shows values of standardized regression coefficient (b) and a relative influence (%) of biological and lifestyle elements on WC, WHtR and BMI. Variation of WHtR was explained in 44% by biological factors i.e. age, the Tanner stage, body height and concentration of leptin and ghrelin as well as by environmental factors i.e. obesity prevalence in fathers and high physical activity of the girls after eliminating influences of the remaining variables included in the analysis. Higher progression of biological development in girls was connected with higher values of the WHtR index, but the older the girls were, the lower values were presented by the index. Also, the taller the girls were, the lower WHtR values were shown. Higher WHtR values were related to higher concentration of leptin in blood serum. Whereas, a reverse direction of dependency was obtained for ghrelin: higher concentration of ghrelin was accompanied by lower WHtR values. Obesity prevalence in fathers, but not mothers, of the examined girls was connected with higher values of the WHtR index. Physically active girls featured lower WHtR values in comparison to the inactive ones. The remaining variables: mothers’ obesity, parents’ education, siblings and regular breakfasts were rejected in the stepwise regression method. More than a half of WHtR variation got explained by biological factors, nevertheless, environmental factors i.e. high level of physical activity also took part in explaining variation of central distribution of adipose tissue (Table 3).
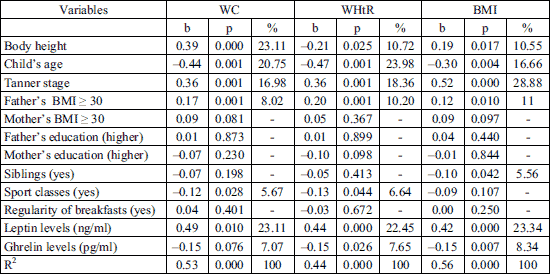
Variation of WC was explained in 53% by a similar set of variables: age, degree of progression in pubescence, body height, leptin and ghrelin concentration, father’s obesity and high physical activity of the girls while eliminating influences of the remaining variables included in the analysis. The dependencies were of a similar direction (apart from body height) as in case of WHtR and the level of leptin in blood serum had the biggest contribution in explaining variation of waist circumference (Table 3).
The BMI variation was explained at the highest level (56%) by the level of biological development progression, leptin concentration, age, body height, ghrelin concentration, obesity prevalence in fathers and a number of children in a family. The dependencies were of a similar direction as for WC. Relations between a level of physical activity and BMI were not confirmed. Moreover, a higher number of children in a family was connected with lower values of BMI (Table 3).
Application of a logistic regression analysis to indicate, from among the analysed variables, the factors which are significantly connected with a risk of abdominal obesity prevalence allowed to confirm a particularly important relation of leptin and abdominal obesity (Table 4). Abdominal obesity (WHtR ≥ 0.50) was proved in only 6% of the examined girls. A risk of exceeding the cut-off points according to WHtR criteria increased by five times with an increase of leptin concentration in blood serum by barely 1ng/ml (OR = 5.24; 95% CI 1.6 – 17.12).
DISCUSSION
An excessive accumulation of adipose tissue is a complex disorder whose pathogenesis involves genetic, biological and environmental factors (20, 21). The conducted analysis confirmed the fact that biological factors such as age, a degree of progression in ontogenesis and levels of leptin and ghrelin are the highest determinants of the adipose tissue distribution type (WHtR and WC) in the examined girls. Nevertheless, apart from the biological factors, a significant role is played by the environmental ones. Attending sport classes with increased number of physical activity hours (swimming training) was connected with lower WHtR and WC values in the examined girls, whereas the girls from regular classes were characterised by a more central type of adipose tissue distribution. Excessive fat distribution in the abdominal region brings consequences resulting from metabolic activity of adipose tissue particularly dangerous for health. Visceral adipose tissue is considered to be more inflammatory than other fat depots, generating pro-inflammatory substances. Therefore, obesity represents a low-grade chronic inflammatory state characterized by abnormal cytokine production and increased synthesis of acute-phase reactants, such as C-reactive protein (CRP) (22). Healthful effect of exercise may, to some extent, be attributed to its the anti-inflammatory effects. Exercise may exert its anti-inflammatory effect via a reduction in visceral fat mass and/or by induction of anti-inflammatory muscle-derived peptides - myokines (22). Application of pharmacotherapy in treating obesity complications is often connected with side effects. For example, statins used in treating hypercholesterolemia induce growth in the size of subcutaneous adipose tissue which worsens insulin resistance in obese Zucker rats (23).
Studies over relations between physical activity and abdominal type obesity confirm that high levels of physical activity are beneficial to prevent abdominal obesity in the paediatric age group (24, 25) and that increased time spent in vigorous physical activities ( > 6 METs) is independently associated with lower waist circumference and visceral fat (26, 27). The examined girls from sport classes regularly undertook physical activity - swimming training, on average 5 ± 1.86 days/week and 2.20 ± 1.07 hours/day and in comparison to girls from regular classes their metabolic equivalent (METs) of energy consumption was two times higher (28). It is suggested that cardiorespiratory fitness (CRF) may be a stronger predictor of abdominal obesity than a habitual activity (25). Lee and Arslanian (29) demonstrated that after accounting for age, sex, and pubertal status, CRF assessed by maximal treadmill test was inversely associated with visceral and abdominal subcutaneous fat in children and adolescents. The studies of Barbeau et al. (30) confirmed that engaging in regular aerobic types of exercise has a protective effect on age-associated increases of visceral fat in growing children.
There is a well-documented age related decline in physical activity, especially as adolescents enter puberty (31). Eisenmann and Wickel (32) listed biological factors contributing to a decrease of physical activity as children age and leptin may influence activity levels. Leptin signals to the brain that fat stores are sufficient and energy intake can decrease while energy expenditure can increase. In lean individuals, normal leptin levels are related to increases in energy expenditure. However in overweight and obese individuals, there is evidence of leptin resistance where serum leptin levels are chronically high, yet do not produce the expected decrease in energy intake and increase in energy expenditure (33). The excessive adipose tissue found in obese individuals results in high circulating leptin levels that saturate the leptin receptors and diminish their ability to regulate energy balance (34). Findings of Romon et al. (35) show that higher circulating leptin concentration is associated with lower levels of physical activity in girls. The results of Belcher et al. (36) suggest that leptin levels at the beginning of puberty may be a salient factor in the steady decline of physical activity levels in girls. In the examined girls the average level of leptin concentration in blood serum increased with age and a degree of progression in ontogenesis. The results of linear and logistic regressions indicate positive dependencies between leptin concentration in blood serum and the central type of adipose tissue distribution. Many factors regulate leptin synthesis and expression such as feeding status, sympathetic activity, exercise, changes in body weight and energy balance (37). Considering a role of leptin in energy expenditure and its response to changes in body composition, exercise training could theoretically be an important modulator of leptin levels. Researchers have studied connections between direct inhibition of leptin release and intensive or continuing exercise. Apart from a decrease in serum leptin levels following particularly acute exercises like an ultramarathon (38), the studies did not find a long lasting reduction in leptin levels after exercise (36, 39). A considerable decrease in fat-free mass caused by exercise is required to decrease leptin secretion before low serum leptin in turn increases food intake (40). The lack of exercise effects contrasts with the effect of strict energy limitation which quickly lowers serum leptin levels before any significant changes in body composition occur (41).
The conducted analysis presents significant negative relations between ghrelin concentration in blood serum and the central type of adipose tissue distribution. The girls featuring higher WHtR and WC values i.e. a more central type of adipose tissue distribution were characterised by lower mean concentration of ghrelin in blood serum compared to the girls of lower WHtR and WC. The obtained results are consistent with reports of other authors, also indicating a negative correlation of ghrelin and fatness indices: % body fat, BMI (42), similarly to leptin concentration (43). Serum concentrations of ghrelin are reduced in states of positive short- and long-term energy balance, as ghrelin is reduced after food intake (44). The regulation of food intake is complex and hypothalamic arcuate nucleus (ARC) plays a crucial role in the integration of the peripheral metabolic signals, including ghrelin. In studies of Pirnik et al. (45) it was shown that there was a response of local ARC tyrosine hydroxylase neurons to peripherally applied growth hormone secretagogue receptor (GHS-R) agonist and antagonist and this response was different in normal and in diet-induced obesity mice.
One of the other factors influencing changes of ghrelin concentration is physical activity (46). The results of tests on the influence of long-term physical effort show that decreases of BMI and the percentage content of adipose tissue are followed by an increase of concentration of total and non-acylated ghrelin, whereas concentration of acylated (active) form does not change (47, 48). The tests show that short term aerobic exercise does not appear to affect total ghrelin (49). Broom et al. (50) demonstrated that exercises on a running track for about an hour lowered concentration of acylated ghrelin for at least 9 hours after the training. Studies of Marzullo et al. (51) show that intense exercises (e.g. aerobics) lowered concentration of acylated ghrelin without affecting total ghrelin concentration. These suppressions of acylated ghrelin appear to be transient, lasting for an hour after exercise and it is possible that they play a role in exercise-induced anorexia (52). It is suggested that application of a diet itself induces an increase of concentration of acylated active isoform of hunger hormone which is balanced by intensified appetite and food intake. A combination of a diet and physical effort brings better effects in body mass control because concentration of acylated (active) ghrelin remains at a steady level and prevents excessive food intake (46).
A conducted multiple regression analysis showed that an increase of the ontogenesis progression degree is followed by increased WHtR values in the examined girls. However, girls tend to get slender with age, a relation of their waist circumference to body height takes values considerably lower than a half of their body height. The phenomenon of getting slender in adolescents is also confirmed by results of former studies including, among others, a population of urban children (53) and rural ones (54). In the process of ontogenesis in children both body height and waist circumference increase and an analysis of WHtR changes accompanying child’s growth reflects mutual relations between the rate of waist circumference growth and body height. It has been suggested that WHtR ≥ 0.50, irrespective of age, sex or ethnicity is a valid predictor of higher cardiometabolic risk (4, 6, 17, 55). However, still the most appropriate cut-off point for WHtR to identify children and adolescents at cardiometabolic risk is not clear. In the present study WHtR ≥ 0.50 was applied although the examined girls were characterised by lower mean values of both waist circumference and the WHtR index in comparison to Cracow population (20, 53) probably resulting from increased physical activity of most of them (sport schools). WHtR may be a more straightforward anthropometric index to apply in the clinical setting where BMI centile charts may not be readily available (55). Apart from that, annual increases in BMI during childhood have been shown to be attributed to increases of lean mass more than increases in fat mass, but varied according to sex and age (56). Freedman and Sherry (57) demonstrated that accuracy of BMI in predicting overweight and obesity varies with degree of fatness, with high accuracy of fat children and lower in thin children. Therefore, changes in BMI percentile do not necessarily reflect changes in adiposity in children over time, especially not in children with lower BMI values (58).
In conclusion, the findings of the present study support the thesis that an abdominal type of adipose tissue distribution in pubertal girls is positively connected with both biological factors such as a degree of ontogenesis progression, leptin level and a variable combining genetic and environmental element i.e. obesity prevalence in the examined girls’ fathers. Negative dependencies were observed between the abdominal type of adipose tissue distribution and age, as well as ghrelin level in blood serum and a high level of physical activity. A general index of adiposity (BMI) did not demonstrate relations with the level of physical activity in young girls. The WHtR seemed to be a more sensitive identifier of environmental behaviours than the general adiposity index. The exact relationship between leptin, ghrelin, physical activity and abdominal type of body fat distribution needs to be further clarified in larger-scale studies.
Acknowledgements: The study was a part of the project no. 214/KF/2006 Suder and Plonka, University of Physical Education, Cracow: ‘Concentration of ghrelin, leptin and growth hormone (GH) in prepubertal girls vs. distribution of adipose tissue in the aspect of differentiated physical activity (longitudinal studies)’.
Conflict of interests: None declared.
REFERENCES
- Malina RM, Koziel S, Bielicki T. Variation in subcutaneous adipose tissue distribution associated with age, sex, and maturation. Am J Hum Biol 1999; 11: 189-200.
- Chrzanowska M, Suder A. Ontogenesis changes and sex dimorphism of subcutaneous fat distribution: 12-year longitudinal study of children and adolescents from Cracow, Poland. Am J Hum Biol 2008; 20: 424-430.
- Goran MI, Gower BA. Relation between visceral fat and disease risk in children and adolescents. Am J Clin Nutr 1999; 70 (Suppl): 149S-156S.
- Bluher S, Molz E, Wiegand S, et al. Body mass index, waist circumference, and waist-to-height ratio as predictors of cardiometabolic risk in childhood obesity depending on pubertal development. J Clin Endocrinol Metab 2013; 98: 3384-3393.
- Kahn H, Imperatore G, Cheng Y. A population-based comparison of BMI percentiles and waist-to-height ratio for identifying cardiovascular risk in youth. J Pediatr 2005: 146: 482-488.
- Garnett SP, Baur LA, Cowell CT. Waist-to-height ratio: a simple option for determining excess central adiposity in young people. Int J Obes (Lond) 2008; 32: 1028-1030.
- Freedman DS, Kahn HS, Mei Z, et al. Relation of body mass index and waist-to-height ratio to cardiovascular disease risk factors in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr 2007; 86: 33-40.
- Schmidt MD, Dwyer T, Magnussen CG, Venn AJ. Predictive associations between alternative measures of childhood adiposity and adult cardio-metabolic health. Int J Obes (Lond) 2011; 35: 38-45.
- Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol 2004; 24: 225-253.
- Mantzoros CS, Magkos F, Brinkoetter M, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab 2011; 301: E567-E584.
- Di Marzo V, Goparaju SK, Wang L, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001; 410: 822-825.
- Kojima M, Hosoda H, Date Y, Nakanzato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999; 402: 656-660.
- Date Y, Kojima M, Hosoda H, et al. Ghrelin- a novel growth hormone-releasing acylated peptide, is synthesised in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000; 141: 4255-4261.
- Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001; 86: 5992.
- Nakazato M, Murakami M, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature 2001; 409: 194-198.
- Perello M, Zigman JM. The role of ghrelin in reward-based eating. Biol Psychiatry 2012; 72: 347-353.
- McCarthy HD, Ashwell M. A study of central fatness using waist-to-height ratios in UK children and adolescents over two decades supports the simple message-“ keep your waist circumference to less than half your height”. Int J Obes (Lond) 2006; 30: 988-992.
- Jebb SA, Cole TJ, Doman D, Murgatroyd PR, Prentice AM. Evaluation of the novel Tanita body-fat analyser to measure body composition by comparison with a four-compartment model. Br J Nutr 2000; 83: 115-122.
- Tanner JM. Rozwoj w okresie pokwitania. PZWL, Warszawa 1963.
- Luczynski W, Zalewski G, Bossowski A. The association of the FTO RS9939609 polymorphism with obesity and metabolic risk factors for cardiovascular diseases in Polish children. J Physiol Pharmacol 2012; 63: 241-248.
- Suder A, Chrzanowska M. Risk factors of abdominal obesity in children and adolescents from Cracow, Poland (1983-2000). J Biosoc Sci 2015; 47: 203-219.
- Bilski J, Mazur-Bialy AL, Wierdak M, Brzozowski T. The impact of physical activity and nutrition on inflammatory bowel disease: the potential role of cross talk between adipose tissue and skeletal muscle. J Physiol Pharmacol 2013; 64: 143-155.
- Aguirre L, Hijona E, Macarulla MT, et al. Several statins increase body and liver fat accumulation in a model of metabolic syndrome. J Physiol Pharmacol 2013; 64: 281-288.
- Saelens BE, Seeley RJ, van Schaick K, Donnelly LF, O’Brien KJ. Visceral abdominal fat is correleted with whole-body fat and physical activity among 8-y-old children at risk of obesity. Am J Clin Nutr 2007; 85: 46-53.
- Kim Y, Lee S. Physical activity and abdominal obesity in youth. Appl Physiol Nutr Metab 2009; 34: 571-581.
- Ortega FB, Ruiz JR, Sjostrom M. Physical activity, overweight and central adiposity in Swedish children and adolescents: the European Youth Heart Study. Int J Behav Nutr Phys Act 2007; 4: 61.
- Dencker M, Thorsson O, Karlsson MK, Linden C, Wollmer P, Andersen LB. Daily physical activity related to aerobic fitness and body fat in an urban sample of children. Scand J Med Sci Sports 2008; 18: 728-735.
- Plonka M, Toton-Morys A, Adamski P, et al. Association of physical activity with leptin blood serum level, body mass indices and obesity in schoolgirls. J Physiol Pharmacol 2011; 62: 647-656.
- Lee SJ, Arslanian SA. Cardiorespiratory fitness and abdominal adiposity in youth. Eur J Clin Nutr 2007; 61: 561-565.
- Barbeau P, Johnson MH, Howe CA, et al. Ten months of exercise improves general and visceral adiposity, bone, and fitness in black girls. Obesity 2007; 15: 2077-2085.
- Pate RR, Stevens J, Webber LS, et al. Age related change in physical activity in adolescent girls. J Adolesc Health 2009; 44: 275-282.
- Eisenmann JC, Wickel EE. The biological basis of physical activity in children: revisited. Pediatr Exerc Sci 2009; 21: 257-272.
- Heptulla R, Smitten A, Teague B, Tamborlane WV, Ma YZ, Caprio S. Temporal patterns of circulating leptin levels in lean and obese adolescents: relationships to insulin, growth hormone, and free fatty acids rhythmicity. J Clin Endocrinol Metab 2001; 86: 90-96.
- Banks WA. The many lives of leptin. Peptides 2004; 24: 331-338.
- Romon M, Lafay M, Bresson JL, et al. Relationships between physical activity and plasma leptin levels in healthy children: the Fleurbaix-Laventie Ville Sante II Study. Int J Obes Relat Metab Disord 2004; 28: 1227-1232.
- Belcher BR, Chou CP, Nguyen-Rodriguez T, et al. Leptin predicts a decline in moderate to vigorous physical activity in minority female children at risk for obesity. Pediatr Obes 2013; 8: 70-77.
- Klok MD, Jakobsdottir S, Drent ML. Te role of leptin and ghrelin in the regulation in food intake and body weight in humans: a review. Obes Rev 2007; 8: 21-34.
- Zaccaria M, Ermolao A, Roi G, Englaro P, Tegon G, Varnier M. Leptin reduction after endurance races differing in duration and energy expenditure. Eur J Appl Phys 2002; 87: 108-111.
- Bouassida A, Chamari K, Zaouali M, Feki Y, Zbidi A, Tabka Z. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br J Sports Med 2010; 44: 620-630.
- Finelli C, Gioia S, La Sala N. Physical activity: an important adaptative mechanism for body-weight control. ISRN Obesity 2012; 2012: 675285.
- Khan SM, Hamnvik OP, Brinkoetter M, Mantzoros CS. Leptin as a modulator of neuroendocrine function in humans. Yonsei Med J 2012; 53: 671-679.
- Siiya T, Nakazato M, Mizuta M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab 2002; 87: 240-244.
- Yoshihara F, Kojima M, Hosoda H, Nakazato M, Kangawa K. Ghrelin: a novel peptide for growth hormone release and feeding regulation. Curr Opin Clin Nutr Metab Care 2002; 5: 391-395.
- Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001; 50: 707-709.
- Pirnik Z, Majercikova Z, Holubova M, et al. Effect of ghrelin receptor agonist and antagonist on the activity of arcuate nucleus tyrosine hydroxylase containing neurons in C57BL/6 male mice exposed to normal or high fat diet. J Physiol Pharmacol 2014; 65: 477-486.
- Adams CE, Greenway FL, Brantley PJ. Lifestyle factors and ghrelin: critical review and implications for weight loss maintenance. Obes Rev 2011; 12: e211-e218.
- Foster-Schubert KE, McTiernan A, Frayo RS, et al. Human plasma ghrelin levels increase during a one-year exercise program. J Clin Endocrinol Metab 2005; 90: 820-825.
- Kim HJ, Lee S, Kim TW, Kim HH, et al. Effects of exercise-induced weight loss on acylated and non-acylated ghrelin in overweight children. Clin Endocrinol 2008; 68: 416-422.
- Burns SF, Broom DR, Miyashita M, Mundy C, Stensel DJ. A single session of treadmill running has no effect on plasma total ghrelin concentrations. J Sports Sci 2007; 25: 635-642.
- Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M. Exercise induced suppression of acylated ghrelin in humans. J Appl Physiol 2007; 102: 2165-2171.
- Marzullo P, Salvadori A, Brunani A, et al. Acylated ghrelin decreases during acute exercise inthe lean and obese state. Clin Endocrinol 2008; 69: 970-971.
- Stensel D. Exercise, appetite and appetite-regulating hormones: implications for food intake and weight control. Ann Nutr Metab 2010; 57 (Suppl. 2): 36-42.
- Chrzanowska M, Suder A. Changes in central fatness and abdominal obesity in children and adolescents from Cracow, Poland 1983–2000. Ann Hum Biol 2010; 37: 242-252.
- Suder A, Janusz M, Jagielski P, et al. Prevalence and risk factors of abdominal obesity in Polish rural children. HOMO 2015; Feb 17. doi:10.1016/j.jchb.2014.09.008.
- Graves L, Garnett SP, Cowell CT, et al. Waist-to height ratio and cardiometabolic risk factors in adolescence: findings from a prospective birth cohort. Pediatr Obes 2014; 9: 327-338.
- Maynard LM, Wisemandle W, Roche AF, Chumlea WC, Guo SS, Siervogel RM. Childhood body composition in relation to body mass index. Pediatrics 2001; 107: 344-350.
- Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics 2009; 124 (Suppl. 1): 23-34.
- Demerath EW, Schubert CM, Maynard LM, et al. Do changes in body mass index percentile reflect changes in body composition in children? Data from the Fels Longitudinal Study. Pediatrics 2006; 117: e487-e495.
A c c e p t e d : February 25, 2015