EMERGING ROLE OF FECAL MICROBIOTA THERAPY IN THE TREATMENT
OF GASTROINTESTINAL AND EXTRA-GASTROINTESTINAL DISEASES
IMPORTANCE OF GUT MICROBIOTA
According to the famous words of Hippocrates, his statement that “all disease begins in the gut” seems to be of great importance nowadays. With these words the father of the modern medicine suggested the essential role played by gut and diet in many vital homeostatic functions of the human body (1). In the recent decade our understanding of the role of the human gut microbiome has been revolutionized by enormous advances in development of molecular methods (especially high throughput DNA sequencing). Based on this knowledge, the gut microbiome is concerned as the most densely populated and diverse microbial communities (2). Approximately, more than 500 different microbial species constituting up to 100 trillion (1014) microorganisms per human that can colonize the intestinal tract making an additional acquired organ weighing ca. 1.5 – 2 kg (Fig. 1). The human gut microbiota is remarkably diverse. The colonization of gut by microbes begins after delivery and the baby early life environment plays a crucial role in the development of healthy microbiome. Once fully developed, gut microbiome becomes the essential acquired organ that provides many vital functions to the host. Currently it has been suggested that 4 different Phyla (Bacteroidetes, Actinobacteria, Firmicutes and Proteobacteria) consisting of thousands of mostly anaerobic species inhabit the human gut with a steep, stomach acid-driven proximal-distal gradient (Fig. 1). The number of genes in microbiota outnumber human genes by one hundredfold (3). The general human population can be stratified on the sole basis of three dominant bacteria (i.e. the concept of enterotypes), or subjects categorization according to their microbiome gene richness. Both aspects have been strengthened by recent studies investigating the impact of nutrients (e.g., dietary fibers, fat feeding) and dietary habits (i.e., vegans versus omnivores) of different populations (4).
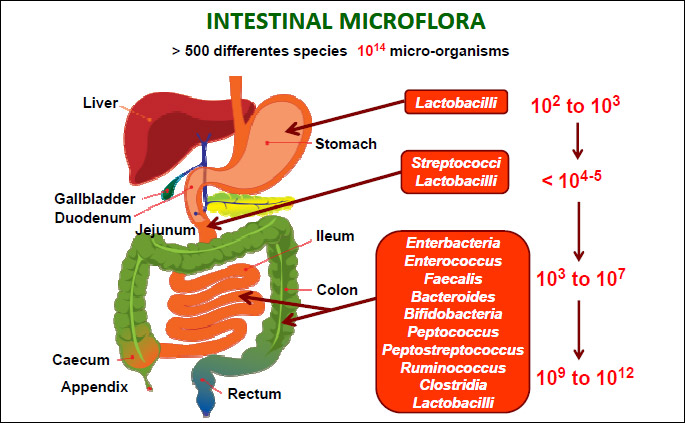
From the clinical point of view the most of the microbial species can be detected by culture independent methods such as 16s ribosomal RNA sequencing method. Importantly, each individual has its own unique microbiota, so the gut microbiome of each individual is like a fingerprint. The composition of the gut microbiota remains relatively stable from late childhood to old age, when changes occur again due to aging process (5, 6). More recently, groups of bacterial families have been classified into enterotypes on the basis of their functions. This classification is based on metabolism of dietary components and ability to tolerate and metabolize drugs which should help to further understanding of the role of enteric microbiota in health and disease. Aging is associated with changes in diversity of non-cultured species, with a greater proportion of Bacteroides species, a distinct abundance of Clostridium clusters, an increased enterobacteria population, and a lower number of Bifidobacteria (7, 8).
The human beings lifestyle, diet and medical interventions including pharmacological therapy including use of antibiotics and proton pump inhibitors have profound impact on the composition (diversity) of the gut microbiota (9). The impairment of homeostasis by dysbiosis may increase susceptibility to inflammatory bowel diseases (IBD) (9). The environment, genetics, and host immunity form a highly interactive regulatory triad that have been shown to regulate toll like receptor (TLR) function. Imbalanced relationships within this triad may promote aberrant TLR signaling, critically contributing to formation of inflammasome resulting in acute and chronic intestinal inflammatory processes, such as in IBD consisting of Crohn disease and ulcerative colitis, and colorectal cancer (10). In particular, IBD are characterized by idiopathic intestinal inflammation that can arise from predisposing genetic (genes encoding proteins relevant to both innate and adaptive immunity: NOD2, STAT3, IL-23 receptor, etc.) and environmental factors (specific TLRs ligands, and antigens derived from commensal bacteria) acting on the immunoregulatory system. It is generally accepted that IBD may be result of an imbalance of proinflammatory- and regulatory-T-cells responses (11, 12).
The “last human organ“ plays a central role in the development of immune system, alterations in intestinal functions and defense against pathogens and metabolism (13) (Fig. 2). Beyond the microbial richness (diversity), a healthy gut microbiome is characterized by the presence of the microbes that enhance metabolism, resilience to infection and inflammation, resistance to cancer and autoimmunity and the positive influence on brain-gut axis (14, 15). Importantly, the gut microbiota affects the intestinal mucosa via interaction with epithelial cells, enteric nervous system leading to changes in gut motility, sensory functions and pain perception (microbiota-brain-gut axis) (16) (Fig. 2). Microbiota-brain interactions constitute an exciting area of research which may contribute to the new insights into individual predispositions in cognition, personality, mood, sleep, and eating behavior (17). Moreover, this interaction can help in understanding of a wide range of neuropsychiatric diseases ranging from affective disorders to autism, depression and schizophrenia (Fig. 2). Gut microbes affect the functions of intestinal barrier, especially its permeability as well as cell proliferation and production of IgA and defensins (18).
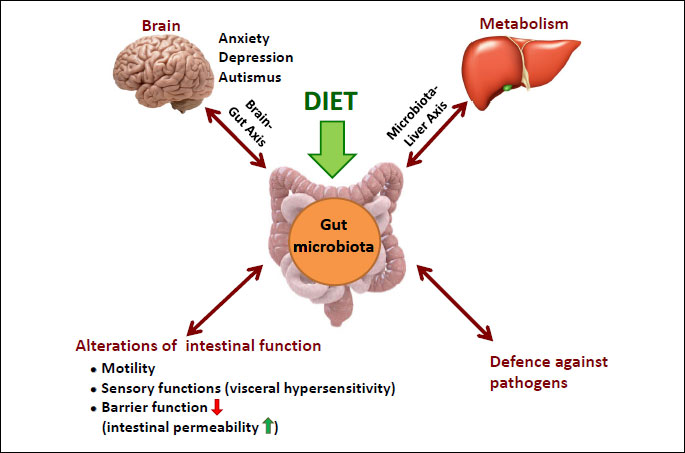
The mechanism by which microbiota may contribute to the function of brain-gut axis remains unknown but microbes release many biologically active substances such as enteric short chain fatty acids (SCFA) that show strong immunomodulatory and sensory effects. Among the SCFAs, the acetic acid (AA), butyric acid (BA), and propionic acid (PPA) are the most aboundant (19). The SCFAs consist of the major class of signaling molecules produced from bacterial fermentation of dietary carbohydrates, odd-chain fatty acids, and some proteins (20, 21). SCFAs act as major energy substrates and directly affect the host digestive tract through phenotypic alteration of colonic epithelial cells. They can act as tumor suppressor agents, in apoptotic cell death, and have recently been shown to be modulators of the enteric neuroendocrine system. SCFAs are also involved in gene regulation of anti-inflammatory processes both in vitro and in vivo (19, 22). Interstingly, SCFAs whose presence in diet has been documented, can be also produced by opportunistic gut bacteria following fermentation of dietary carbohydrates. They are considered to act as environmental triggers in autism spectrum disorders. With other words, microbes and their metabolites regulate not only the local mucosal immune system through both pro-inflammatory and anti-inflammatory mechanisms but can exert a central effects in brain system (9, 23) (Fig. 2).
One of the most important factors influencing the composition and function of the microbiota is our diet. The type of diet may have great influence on the composition of the gut microbiome. So it is not surprising that westernized diet that is high in sugar and fat leads to the impairment of the gut microbiota termed dysbiosis (24). The data in rodents have shown that within the first week of ingestion of a high fat diet, a significant increase in paracellular permeability in the small intestine has been observed. These permeability changes were accompanied by expression of IL-10 and the genera in the class Clostridia significantly correlated with these early onset changes (25). In contrast, transcellular flux increased in the large intestine and at time points later than first week. These changes correlated significantly with genera in the order Bacteriodales and coincided with increased adiposity, body weight, and plasma levels of lipopolysaccharide-binding protein, together with increased expression of IL-1β in the ileum. Moreover, a positive correlation between proinflammatory cytokine expression in the ileum (IL-1β) and adiposity was observed. Interstingly, a decrease in anti-inflammatory gene expression (IL-10) preceded the increase in the proinflammatory cytokine expression. This was corroborative with the observation that IL-10 knockout mice have increased intestinal permeability before the onset of microbiota-induced gut inflammation (26).
The perturbation of the gut microbiota can be initiated by a number of extrinsic and intrinsic factors. The consequence of this perturbations is the movement from normobiosis (healthy gut) to dysbiosis as documented by less richness of microbiota and its diversity, negative of both, compositional and functional features. This impairment of gut microbiota has been associated with the development of number of diseases in the gastrointestinal (GI) tract and beyond GI tract (27).
According to recent advances in microbiome research, the infectious, inflammatory and functional bowel diseases are closely associated with the pathologic changes in gut microbiota. Beyond the gut, especially the metabolic diseases and neurological disorders due to dysfunction of brain-gut axis and dysbiosis have been linked with the pathology within the gut as manifested by the sensory dysfunction and intestinal permeability thus predisposing to gastrointestinal diseases (28). One of the important discoveries in the last years is the fact that the disbalance of gut microbiome has a profound impact on the function of the liver via microbiota-liver axis (Fig. 2). The mechanisms through which microbes and their metabolites affect the metabolic homeostasis are just at the beginning to be understood. One of the consequences of dysbiosis is “leaky gut” i.e. increased gut permeability and increased microbial translocation through the mucosal barrier leading to metabolic endotoxemia and subsequent low-grade inflammation. The consequence of this phenomenon is the increased generation of proinflammatory cytokines and free radicals leading to inflammation in the liver or pancreas. In contrast, healthy microbiota is an important factor in the prevention of metabolic dysregulation (29).
Leaky gut and dysbiosis have been linked with the development of non-alcoholic liver disease and nonalcoholic steatohepatitis as well as diabetes mellitus and metabolic syndrome (30). The member of new generation of antibiotic designed to treat lower bowel disorders such as rifaximin exerts a multifaceted mechanism of action that include a direct antimicrobial effect, the inhibition of bacterial translocation across the gut mucosal epithelium and subsequent alteration in the release and/or absorption of endotoxin and bacterial metabolites, and modulation of gut-immune signaling (31).
MICROBIOTA AND AGING
The relationship exist between the microbiota and a variety of clinical problems associated with erderly, including physical frailty, Clostridium difficile (C. difficile) colitis, vulvovaginal atrophy, colorectal carcinoma, and atherosclerotic disease. The stratification of the proper composition of microbiota and microbiome of older adults holds promise to work out as an innovative strategy against the development of comorbidities associated with aging (32). This aging process, affecting many aspects of gut physiology (erderly change of diet, hypochlorhydria, use of drugs, motility disorders), has a profound effect on the composition, diversity and functional features of gut microbiota (Fig. 3). The main gut bacterial phyla, in the order of numerical importance, are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Verrucomicrobia and Fusobacteria (32). Firmicutes are gram-positive bacteria including the large class of Clostridia and the lactic acid bacteria, while Actinobacteria are gram-positive bacteria, including Colinsella and Bifidobacterium spp. The lactic acid bacteria and Bifidobacteria are two important types of gut bacteria, which are autochthonous ones from birth or acquired from digested food. Lactobacillus and Leuconostoc spp. are the main lactic acid bacteria found in the human intestine. Bifidobacterium spp. is the predominant bacteria found among the first colonizers of newborns, and persists at a low level in adults (34). Interestingly, in aging the number of Firmicutes increases while the number of SCFA-producing Bacetroidetes significantly decreases (35, 36) (Fig. 3).
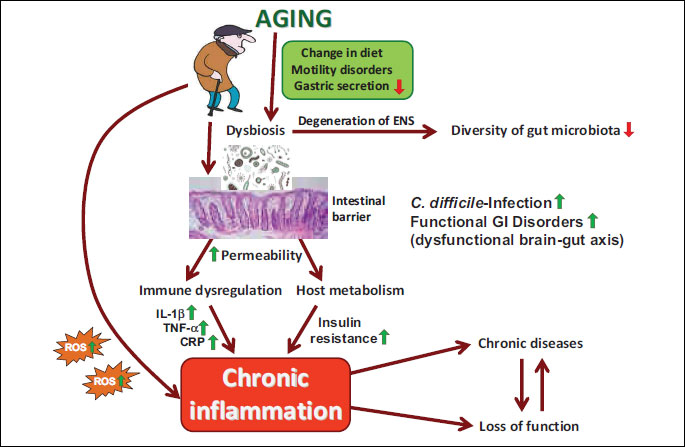
Due to aging process the diversity (richness) decreases and the propensity to develop the pathological C. difficile infection significantly increases along with immune dysregulation followed by the abundance of local and systemic proinflammatory markers (IL-1β, TNF-α and CRP) (Fig. 3). Additionally, the increasing permeability of gut barrier due to dysbiosis may increase the risk of developing numerous chronic diseases (37) (Fig. 3). The effect of aging process on the gut microbiota composition is nowadays receiving a considerable interest, especially from the perspective of improving the wellness of aged people by modulating the gut microbiota in elderly (38). On the other hand, the link between gut microbiota and the aging process is only partially understood, with the gut ecosystem exhibiting the potential to become a promising target for strategies considered to improve the health status of aging population (39).
FECAL MICROBIOTA THERAPY AND C. DIFFICILE INFECTION
Based on existing evidence it is not surprising that C. difficile-induced colitis is one of the emerging infectious diseases of the GI tract in elderly patients. The intestinal inflammation caused by C. difficile is characterized by diarrhea, pseudomembranous colitis and sometimes complicated by life-threading fulminate colitis. C. difficile colitis is a serious complication of antibiotic use and prolonged hospitalization. Based on huge number of studies, the impairment of microbiota and its decreased diversity appear to be the major factors in the pathogenesis of C. difficile. Besides the use of antibiotics, other risk factors such as increased age (> 65 years), comorbidity or use of drugs (especially proton pump inhibitors) are responsible for the decrease of microbiota diversity and increased predisposition to the development of this disease. From the clinical point of view C. difficile colitis is characterized by increasing incidence, complication and mortality rates. This phenomenon is partly due to bacterial virulence factors and production of bacterial toxins. This disease is associated with increased length of hospitalization and therapy costs. C. difficile infection shows recurrent character and the development of recurrences increase the resistance to antibiotic therapy after each episode of C. difficile infection (40-42).
The main problem of C. difficile infection in the clinical practice results from its recurrent character. Despite appropriate antibiotic treatment, some patients develop chronic recurrent C. difficile infection. The development of recurrences increases after each episode of C. difficile infection. A recurrent episode of C. difficile infection may be result of incomplete eradication of the offending strain and its spores or could reflect reinfection with new pathogenic strain (43). The initial therapy against C. difficile conventionally consists of antibiotics such as metronidazole or vancomycin. Unfortunately, the antibiotic therapy of the initial episode of C. difficile infection fails in 19 – 30% of cases. Recently, more effective novel antibiotic fidaxomycin has been successfully introduced. After first recurrence, fidaxomycin was more effective than vancomycin and the cure-rate in the first group was 76% compared to 59% in the vancomycin group. Fidaxomycin is almost non-absorbable from GI tract and less disturbing to gut microbiota as compared to vancomycin. Despite these advances in the antibiotic therapy of C. difficile, the effectiveness of the antibiotic therapy after its each refractory episode decreases dramatically (up to 30%). In patients with multiple relapses, the effectiveness of all antibiotic approaches including fidaxomycin is significantly reduced and other treatment modalities are definitely needed (44).
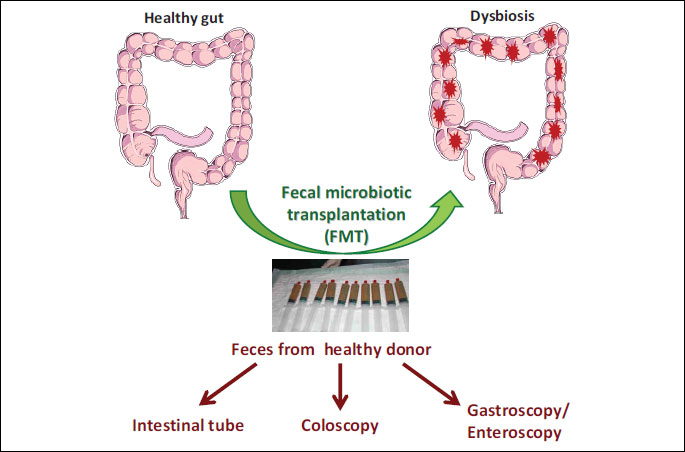
The majority of data indicating that healthy microbiota is required for human health and protects against disease, have focused so far on microbiota-induced therapeutic potential and possible clinical interventions based on delivery of probiotic microbiota and compounds containing probiotics. Moreover, the fecal microbiota transplantation (FMT) has been shown to be much more effective than antibiotics against recurrent or refractory C. difficile colitis (45). FMT refers to the transfer of feces obtained from the healthy donor into the patient’s gastrointestinal tract via different routes (gastric, jejunal or colonoscopic route) (Fig. 4). Currently, there is no consensus which is the best delivery method for the infusion of feces. In contrast to antibiotics, FMT is the only method that has been reported to restore the intestinal homeostasis and prevent the recurrence of C. difficile infection (46, 47).
The first published evidence on introducing FMT was published by Eiseman et al (48), but historically this method was already known in the ancient China. Despite effectiveness of this method, fecal material has potential risks for the recipient as a treatment with the use of an active biological material, so this treatment is still considered as premature in terms of mechanism of action and the consequences that it may cause. Each donor must be screened for infectious diseases (HIV, hepatitis, C. difficile. etc). Besides laboratory screening, each donor is additionally screened based on history including recent antibiotic therapies, use of immunosuppressive drugs, exposure to HIV or hepatitis, history of allergic, inflammatory, metabolic or malignant disease. It is of great importance that donor of feces should be as healthy as possible. There is a hope that FMT may eventually prove beneficial for the treatment of other diseases associated with alterations in gut microbiota such as IBD, irritable bowel syndrome (IBS) and metabolic syndrome. Although the basic principles that underlie the mechanisms by which FMT shows therapeutic efficacy in CDI are becoming apparent, further research is still needed to understand the possible role of FMT in the therapy of not only CDI patients but also other GI and non-GI disorders (49).
There are numerous routes available by which FMT can be administered (Fig. 4). The advantage and disadvantage of each method of application is different. The FMT via nasogastric/nasojejunal tube is minimally invasive and considered as a low cost procedure. The disadvantage for the patients is the increased risk of aspiration and vomiting. The use of gastroscopy or enteroscopy may overcome some of these problems, but these methods are invasive, require sedation and are associated with higher costs. The most common way to application the FMT is colonoscopy. The main advantage of this method is the fact that it is more appealing to patients and probably more effective in the therapy of CDI. In addition, the colonoscopy enables the direct evaluation of the lower tract and exclusion of other pathologies (Fig. 4). The disadvantage of this method are higher costs, need of sedation and risks of complications due to colonoscopy (perforations, bleeding and others). Finally, the use of stool in a manufactured prepared capsule from a standard screened donor represents an non-invasive method for FMT that could minimize the obviates risk and cost of endoscopy and donor screening. However, the effectiveness of this type of application need to be assessed in further prospective studies.
The effective amount of feces required for FMT is still not standardized, but amount between 50 – 100 g seems to be sufficient for the successful microbiota transfer. Normally, the fresh fecal preparation is used for FMT. However, the recent study by Satokari (50) demonstrated that the use of the frozen fecal inoculums from a standard donor can be applied for FMT without the loss of efficacy. Frozen feces preparation showed similar efficacy as fresh feces in treating recurrent C. difficile. infection (51). The first randomized trial with FMT in patients with recurrent CDI was performed by Van Nood group that demonstrated high effectiveness of this method in the treatment of recurrent CDI-induced colitis (52). More important, FMT led to the restoration of intestinal homeostasis and increased microbial diversity (52, 53).
One of the important challenges in the practical use of FMT is the instinctive aversion to fecal material and risk of infection. To minimize these risks, scientist are working on synthetic stool. The development of stool banks from safe donors could be the other alternative (54, 55).
The side effects of FMT are low and include risk of acute infections (biosafety), allergic reactions, potential transmission of bacteria that increase the predisposition to chronic diseases including IBD, IBS, colorectal cancer, diabetes mellitus type 2 or metabolic syndrome (56). The only recently published the case of unintentional weight gain after FMT supports the concept that microbiota may transfer metabolic phenotype (57). Finally, the patients with IBD with the impairment of intestinal barrier were reported to have fever or Escherichia coli-induced bacteremia after implantation of FMT. The data on long-term side effects of FMT is still limited. Importantly, a recently published evidence on the treatment with FMT in immune-compromised patients demonstrated that this method is useful and safe in these patients (58).
FECAL MICROBIOTA THERAPY AND INFLAMMATORY BOWEL DISEASE
Gut microbiota impairment plays a fundamental role in the pathogenesis of IBD. The rationale and further indications for FMT to treat IBD could be due to the fact that this disease is characterized by the pathologic immune response to bacteria or presence of pathologic bacteria or due to both phenomenon’s. Numerous studies have shown that IBD is associated with loss in the richness and diversity (dysbiosis) of the gut microbiome. The typical dysbiotic feature is the under-representation of the anti-inflammatory phyla Bacteroides und Firmicutes and relative increase in pro-inflammatory proteobacteria (59). The results of FMT in IBD patients show very promising but also discrepant results. The recent meta-analysis by Colman et al (60) included eighteen studies. Overall, 45% of patients achieved clinical remission and reduced some anti-inflammatory drugs after FMT (60). In the recent randomized trial (61) patients with ulcerative colitis were assigned to FMT treatment with own feces or feces from a healthy donor administered by nasojejunal tube. Seven out of 23 patients (30.4%) in the donor arm versus five out of 25 patients in placebo arm (20%) achieved remission, however, the difference was not statistical significant. Interestingly, in all responders a microbiotic shift in microbiota composition was observed supporting the role of microbiota manipulation in the treatment of IBD (61). Concerning the role of FMT in the management of IBD, there are still many open questions concerning the FMT therapy in IBD patients including the route of administration, frequency of application, microbiota screening in the donor, preparation of the donor stool, administration of antibiotics to the FMT recipient, and many others.
FECAL MICROBIOTA THERAPY AND FUNCTIONAL GASTROINTESTINAL DISORDERS
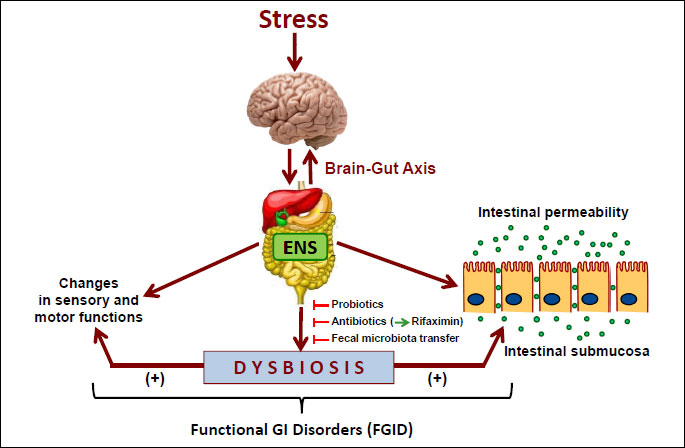
One of the promising therapeutic target for FMT are functional GI disorders (FGID). Numerous studies have revealed that these commonly diagnosed diseases such as IBS may develop due to the dysfunction of brain-gut axis evoked by stress leading to the alterations in the mucosal inflammatory response, visceral pain reception, colonic motility and alterations in behavior. There are several lines of evidence supporting the role of gut microbiota in the pathogenesis of FGID including the differences in intestinal microbiota composition between healthy subjects and patients with IBS, the development of IBS after gastroenteritis (post-infectious IBS), the efficacy of certain antibiotics (like rifaximin) and pre- or probiotics in IBS patients and additional evidences from animal studies (Fig. 5). FGID are accompanied by dysbiosis indicating that the restoration of intestinal homeostasis by FMT could have a positive effect on the natural course of these diseases (62, 63). All these observations indicate that the gut microbiota represents an important therapeutic target in stress-dependent GI dysfunctions (Fig. 5). Recent studies, including our own demonstrated that the modulation of microbiota with probiotics or antibiotics may ameliorate the symptoms of IBS and intestinal inflammation in rodent model of colitis (64). Moreover, the animal studies in germ free mice indicate that the lack of gut microbiota may have impact on memory anxiety, sociability and the release of neurotransmitters such as BDNF supporting the importance of brain-microbiota axis (65, 66). It should be stressed, however, that the number of studies with FMT in FGID is very limited and the placebo-controlled studies are still lacking, but the first results are quite promising and warrant further evaluation (67).
FECAL MICROBIOTA THERAPY AND METABOLIC DISORDERS
In addition to intestinal microbiota alterations leading to GI diseases, the manifestations of extra GI diseases include obesity and metabolic disorders, both associated with multiple perturbations of gut microbiota (68). Some studies indicate that the obesity is associated with lower microbial diversity as manifested by the shift in intestinal microbial flora e.g. an increase in Firmicutes and a decrease in Bacteroides. It was also demonstrated that microbiota in obese subjects is more effective in harvesting energy from food. The adverse role of pathogenic microbial strains in the obesity is further supported by animal studies because the transplantation of gut microbiota from obese to germ-free mice led to an increase in weight gain. The another evidence of contribution of pathogenic gut microbiota to the development of obesity is the fact that vancomycin therapy resulted in decreased peripheral insulin sensitivity in patients with metabolic syndrome (69). As mentioned in this review, the microbiota produce a wide range of metabolites (e.g. SCFA) that may exert a profound effect on the host metabolism being the energy source for epithelial cells and play an important triggering role for the intestinal immune system. Finally, the bariatric surgeries for obesity have been shown to change the composition of microbiome (68-70). The first preliminary pilot study in humans demonstrated that FMT from lean to obese subject improved the insulin resistance due to compositional changes of gut microbiota and increased number of SCFA-producing bacteria (71).
In summary, we conclude that change in microbial composition (dysbiosis) can be implicated in the increasing propensity for a broad range of gastrointestinal and non-gastrointestinal diseases. The gut microbiota represents an important acquired organ, executing numerous vital functions in the metabolism, the development of immune system and the host defense against pathogens. Based on recent evidence in severe and complicated C. difficile infected patients, the novel treatment FMT with or without selected use of vancomycin (72) leads to a successful treatment of C. difficile infection possibly due to increased gut microbiota diversity originally manifested by a “shift” in microflora i.e. an increase in Firmicutes and a decrease in Proteobacteriae. The FMT shows promising results in patients with IBD but this approach still should be further explored by basic and clinical investigators to finally confirm the ultimate effectiveness of this method in the treatment of this disease. Preliminary studies have indicated that the intestinal microbiota by means of FMT could be considered as an important “physiologic” factor in the prevention and treatment of metabolic dysregulation, effective in improving of the insulin resistance and metabolic syndrome. Finally, the aberrant gut microbiota is involved in the pathogenesis of FGID, and affects peripheral and central pathways involved in motility, immunity and brain gut-communication. Thus, the counteracting effect and restoration of the intestinal homeostasis by FMT holds promise that this procedure might also be effective in treatment of FGID.
Conflict of interests: None declared.
REFERENCES
- Savel RH, Munro CL. From Asclepius to Hippocrates: the art and science of healing. Am J Crit Care 2014; 23: 437-439.
- Hollister EB, Gao C, Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology 2014; 146: 1449-1458.
- Power SE, O’Toole PW, Stanton C, Ross RP, Fitzgerald GF. Intestinal microbiota, diet and health. Br J Nutr 2014; 111: 387-402.
- Cani PD, Everard A. Talking microbes: when gut bacteria interact with diet and host organs. Mol Nutr Food Res 2015; Jul 16: doi: 10.1002/mnfr.201500406 (epub ahead of print).
- Petschow B, Dore J, Hibberd P, et al Probiotics, prebiotics, and the host microbiome: the science of translation. Ann NY Acad Sci 2013; 1306: 1-17.
- Sun J, Chang EB. Exploring gut microbes in human health and disease: Pushing the envelope. Genes Dis 2014; 1: 132-139.
- Arumugam M, Raes J, Pelletier E, et al Enterotypes of the human gut microbiome. Nature 2011; 473: 174-180.
- Claesson MJ, Cusack S, O’Sullivan O, et al Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA 2011; 108 (Suppl. 1): 4586-4591.
- Simren M. IBS with intestinal microbial dysbiosis: a new and clinically relevant subgroup? Gut 2014; 63: 1685-1686.
- Frosali S, Pagliari D, Gambassi G, Landolfi R, Pandolfi F, Cianci R. How the intricate interaction among Toll-like receptors, microbiota, and intestinal immunity can influence gastrointestinal pathology. J Immunol Res 2015; 2015: 489821.
- Pandolfi F, Cianci R, Pagliari D, Landolfi R, Cammarota G. Cellular mediators of inflammation: tregs and TH17 cells in gastrointestinal diseases. Mediators Inflamm 2009; 2009: 132028.
- Geremia A, Biancheri, Allan P, Corazza GR, di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev 2014; 13: 3-10.
- Goulet O. Potential role of the intestinal microbiota in programming health and disease. Nutr Rev 2015; 73 (Suppl. 1): 32-40.
- Lankelma JM, Nieuwdorp M, de Vos WM, Wiersinga WJ. The gut microbiota in internal medicine: implications for health and disease. Neth J Med 2015; 73: 61-68.
- Schippa S, Conte MP. Dysbiotic events in gut microbiota: impact on human health. Nutrients 2014; 6: 5786-5805.
- Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol 2011; 62: 591-599.
- Burokas A, Moloney RD, Dinan TG, Cryan JF. Microbiota regulation of the mammalian gut-brain axis. Adv Appl Microbiol 2015; 91: 1-62.
- Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil 2012; 24: 405-413.
- Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta 2010; 1801: 1175-1183.
- Zoetendal EG, de Vos WM. Effect of diet on the intestinal microbiota and its activity. Curr Opin Gastroenterol 2014; 30: 189-195.
- Heerdt BG, Houston MA, Augenlicht LH. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines. Cancer Res 1994; 54: 3288-3293.
- Heerdt BG, Houston MA, Augenlicht LH. Short-chain fatty acid-initiated cell cycle arrest and apoptosis of colonic epithelial cells is linked to mitochondrial function. Cell Growth Differ 1997; 8: 523-532.
- Shoaie S, Nielsen J. Elucidating the interactions between the human gut microbiota and its host through metabolic modeling. Front Genet 2014; 5: 86.
- Tilg H, Moschen AR. Food, Immunity, and the microbiome. Gastroenterology 2015; 148: 1107-1119.
- Hamilton MK, Boudry G, Lemay DG, Raybould HE. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am J Physiol Gastrointest Liver Physiol 2015; 308: G840-G851.
- Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis 1999; 5: 262-270.
- Mondot S, de Wouters T, Dore J, Lepage P. The human gut microbiome and its dysfunctions. Dig Dis 2013; 31: 278-285.
- DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol 2011; 8: 523-531.
- Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology 2015; 148: 30-36.
- Mehal WZ. The Gordian Knot of dysbiosis, obesity and NAFLD. Nat Rev Gastroenterol Hepatol 2013; 10: 637-644.
- DuPont HL. Therapeutic effects and mechanisms of action of rifaximin in gastrointestinal diseases. Mayo Clin Proc 2015; 90: 116-1124.
- Zapata HJ, Quagliarello VJ. The microbiota and microbiome in aging: potential implications in health and age-related diseases. J Am Geriatr Soc 2015; 63: 776-781.
- Hermann-Bank ML, Skovgaard K, Stockmarr A, Larsen N, Molbak L. The gut microbiotassay: a high-throughput qPCR approach combinable with next generation sequencing to study gut microbial diversity. BMC Genomics 2013; 14: 788.
- Vaughan EE, Heilig H, Ben-Amor K, de Vos WM. Diversity, vitality and activities of intestinal lactic acid bacteria and bifidobacteria assessed by molecular approaches. FEMS Microbiol Rev 2005; 29: 477-490.
- Rehman T. Role of the gut microbiota in age-related chronic inflammation. Endocr Metab Immune Disord Drug Targets 2012; 12: 361-367.
- Mariat D, Firmesse O, Levenez F, et al The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol 2009; 9: 123.
- Keller JM, Surawicz CM. Clostridium difficile infection in the elderly. Clin Geriatr Med 2014; 30: 79-93.
- Biagi E, Candela M, Franceschi C, Brigidi P. The aging gut microbiota: new perspectives. Ageing Res Rev 2011; 10: 428-429.
- Biagi E, Candela M, Turroni S, Garagnani P, Franceschi C, Brigidi P. Ageing and gut microbes: perspectives for health maintenance and longevity. Pharmacol Res 2013; 69: 11-20.
- Keller JJ, Kuijper EJ. Treatment of recurrent and severe Clostridium difficile infection. Annu Rev Med 2015; 66: 373-86.
- Korman TM. Diagnosis and management of Clostridium difficile infection. Semin Respir Crit Care Med 2015; 36: 31-43.
- Czepiel J, Biesiada G, Brzozowski T, et al The role of local and systemic cytokines in patients infected with Clostridium difficile. J Physiol Pharmacol 2014; 65: 695-703.
- Bouza E. Consequences of Clostridium difficile infection: understanding the healthcare burden. Clin Microbiol Infect 2012; 18 (Suppl. 6): 5-12.
- Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA 2015; 313: 398-408.
- Tacke D, Wisplinghoff H, Kretzschmar A, et al First implementation of frozen, capsulized faecal microbiota transplantation for recurrent Clostridium difficile infection into clinical practice in Europe. Clin Microbiol Infect 2015; Jul 7: S1198-743X(15)00689-8 (epub ahead of print).
- Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology 2013; 145: 946-953.
- Brandt LJ. American Journal of Gastroenterology Lecture: Intestinal microbiota and the role of fecal microbiota transplant (FMT) in treatment of C. difficile infection. Am J Gastroenterol 2013; 108: 177-185.
- Eiseman B, Silen W, Bascom CS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44: 854-859.
- Kelly CR, Kahn S, Kashyap P, et al Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology 2015; 149: 223-237.
- Satokari R, Mattila E, KainulainenV, Arkkila PE. Simple faecal preparation and efficacy of frozen inoculum in faecal microbiota transplantation for recurrent Clostridium difficile infection - an observational cohort study. Aliment Pharmacol Ther 2015; 41: 46-53.
- Koenigsknecht MJ, Young VB. Faecal microbiota transplantation for the treatment of recurrent Clostridium difficile infection: current promise and future needs. Curr Opin Gastroeuterol 2013; 29: 628-632
- van Nood E, Vrieze A, Nieuwdorp M, et al Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368: 407-415.
- Song Y, Garg S, Girotra M, et al Microbiota dynamics in patients treated with fecal microbiota transplantation for recurrent Clostridium difficile infection. PLoS One 2013; 8: e81330.
- Petrof EO, Gloor GB, Vanner SJ, et al Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome 2013; 1: 3.
- Petrof EO, Khoruts A. From stool transplants to next-generation microbiota therapeutics. Gastroenterology 2014; 146: 1573-1582.
- Rao K, Young VB. Fecal microbiota transplantation for the management of Clostridium difficile infection. Infect Dis Clin North Am 2015; 29: 109-122.
- Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis 2015; 2: ofv004. doi: 10.1093/ofid/ofv004.
- Kelly CR, Ihunnah C, Fischer M, et al Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol 2014; 109: 1065-1071.
- Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol 2015; 37: 47-55.
- Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis 2014; 8: 1569-1581.
- Rossen N, Fuentes S, van der Spek M, et al Findings from randomized controlled trial of faecal transplantation for patients with ulcerative colitis. Gastroenterology 2015; 149: 110-118.
- Shanahan F, Quigley EM. Manipulation of the microbiota for treatment of IBS and IBD-challenges and controversies. Gastroenterology 2014; 146: 1554-1563.
- Ringel Y, Maharshak N. Intestinal microbiota and immune function in the pathogenesis of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2013; 305: G529-G541.
- Dylag K, Hubalewska-Mazgaj M, Surmiak M, Szmyd J, Brzozowski T. Probiotics in the mechanism of protection against gut inflammation and therapy of gastrointestinal disorders. Curr Pharm Des 2014; 20: 1149-1155.
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconcious: how gut microbes shape human behaviour. J Psychiatr Res 2015; 63: 1-9.
- Siuda D, Wu Z, Chen Y, et al Social isolation-induced epigenetic changes in midbrain of adult mice. J Physiol Pharmacol 2014; 65: 247-255.
- Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation (FMT) an effective treatment for patients with functional gastrointestinal disorders (FGID)? Neurogastroenterol Motil 2015; 27: 19-29.
- Fierbinteanu-Braticevici C. Negreanu L, Tarantino G. Is fatty liver always benign and should not consequently be treated? J Physiol Phamacol 2013; 64: 3-9.
- Parekh PJ, Arusi E, Vinik AI, Johnson DA. The role and influence of gut microbiota in pathogenesis and management of obesity and metabolic syndrome. Front Endocrinol (Lausanne) 2014; 5: 47.
- Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest 2011; 121: 2126-2132.
- Vrieze A, Van Nood E, Holleman F, et al Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012; 143: 913-916.
- Fischer M, Sipe, BW, Rogers NA, et al Faecal microbiota transplantation plus selected use of vancomycin for severe-complicated Clostridium difficile infection: description of a protocol with high success rate. Aliment Pharmacol Ther 2015; 42: 470-476.
A c c e p t e d : July 24, 2015