PROTECTIVE EFFECTS OF VORTIOXETINE IN PREDATOR SCENT STRESS MODEL OF POST-TRAUMATIC STRESS DISORDER IN RATS:
ROLE ON NEUROPLASTICITY AND APOPTOSIS
INTRODUCTION
Post-traumatic stress disorder (PTSD) is characterized by hyperarousal and recurrent stressful memories related to emotional trauma (1). PTSD has been associated with anxiety, cognitive and memory deficits (2-4).
Amygdala, hippocampus, and prefrontal cortex (PFC) are the most affected brain regions in PTSD (5). Subjects with PTSD exert, hypo-active PFC and hippocampus, while hyper-active basolateral amygdala (BLA) (6). The presence of GABAergic interneurons in the entire amygdala is well known and these neurons are important to provide inhibitory control over the amygdala (5).
Both physical stresses for example, diabetic neuropathy (7) and psychological traumatic experiences change neuronal morphology, function, and neurochemistry (1). The presence of abnormal apoptosis including the hippocampus, amygdala, and prefrontal cortex has been reported in various PTSD rat models (8-10). Neuronal apoptosis is related to mitochondria-mediated apoptosis factors, such as caspase-3 (casp-3), caspase-9, and bcl-2 associated X protein (bax) (9, 10). Stressful experiences such as exposure to predator scent, cause increasing of levels of circulating adrenal steroids. Increased adrenal-glucocorticoids, inhibit cell production in the hippocampal dentate gyrus in rats (11) and reduces bcl-2 levels, and this effect causes increased sensitivity to excitotoxic damage (12). Predator scent stress (PSS) causes neuronal damage and increased apoptosis in the hippocampus (13, 14).
Due to stress, a decrease in brain-derived neurotrophic factor (BDNF) level, an increase in glutamate and cortisol levels, and inhibition of neurogenesis may occur in the hippocampus (15). Experimental PTSD models showed that BDNF levels decreased; in the hippocampus (16-18) prefrontal cortex (19), and amygdala (17-20). In contrast, some studies have indicated that increased BDNF levels (16) and the steady-state BDNF levels in BLA, PFC, and hippocampus (21).
Exposure to a PSS model is a commonly used model for studying PTSD (22, 23). In the acute PSS model, rats are exposed to a reminder one week after odour stress procedure. After the reminder, anxiety, and activity evaluate by elevated plus maze (EPM) and hole board test (HBT) while working memory evaluates with a novel object recognition test (NORT) (22-24). Inescapable exposure to predator odor induces persistent alterations in behavioral and physiological responses in rats that imitate the symptom profile of fear and anxiety in PTSD (25). This model produces significant physiological and behavioural alterations including increased anxiety (26), psychological stress (22), cognitive impairment (26), and meets the expectations of many of the diagnostic criteria of DMS-V for PTSD (27).
Numerous therapy approaches have been proposed to cure the PTSD patients (28). Selective serotonin reuptake (SSRI) has been approved for the treatment although remission of PTSD is around 30% with them (29). Animal studies have reported that treatment before stress with antidepressants reduce behavioral deficits related to stress. Antidepressants, including both norepinephrine and SSRIs, promote nerve growth (neurogenesis) in the hippocampus (30, 31) and cortex (31) while stress inhibits neurogenesis. On the other hand, the drugs currently do not fully improve the symptoms of PTSD and they have side effects (3, 29). Therefore, it is really essential to examine the underlying biological mechanisms in PTSD and it is worth investigating to develop new strategies in order to ameliorate the PTSD that could be employed in this regard.
Vortioxetine (Vrx), as a novel serotonergic antidepressant with multimodal activity, shows both antidepressant and anxiolytic effects (32, 33). Vrx is an antagonist of 5-HT1D, 5-HT3, 5-HT7 receptors, an agonist of 5-HT1A receptors, a partial agonist of 5-HT1B receptors, and an SSRI inhibiting the serotonin transporter (SERT) (32, 34). The preclinical and the clinical studies suggest that 5-HT3 and 5-HT7 receptor antagonism and 5-HT1A receptor agonism may have a positive impact on the cognitive functions (33). Furthermore, it has been reported that Vrx can improve cognitive function through the modulation of different neurotransmitters (35) and potentially enhance memory (36). Vrx could modulate several preclinical parameters recognized to be involved in cognitive functions. Vrx prevented the effect of stress on hippocampal LTP, increased rapidly hippocampal cell proliferation and enhanced short-term recognition memory in rats via its 5-HT3 receptor antagonism (37). Vrx increased hippocampal BDNF levels in rats subject to chronic unpredictable mild stress (38).
There is extensive literature showing the efficacy of Vrx on generalized and social anxiety disorder (39-41), however, it is difficult to find studies that examine Vrx and PTSD. Vrx has been approved for the treatment of depression however, no approved by the Food and Drug Administration for the treatment of PTSD. A phase IV clinical trial is currently being conducted for the treatment of PTSD with Vrx (https://clinicaltrials.gov/ct2/show/NCT02637895).
This study aimed to investigate the possible protective effects of Vrx on PTSD model in terms of cognitive and behavioural functions, neuroplasticity and apoptotic mechanisms. In this study, it has been hypothesized that Vrx has beneficial effects on PTSD may be specifically related to the level of BDNF, mitochondria-mediated apoptosis factors; pro- (bax, caspase-3, and caspase-9) and anti-apoptotic (bcl-2) proteins in the amygdaloid complex, the hippocampus, and the frontal cortex.
MATERIAL AND METHODS
Animals and conditions
All experimental procedures involving animals were performed in accordance with the NIH guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). An approval from the institutional ethical committee was obtained from Marmara University Animal Care and Use Committee (MUHDEK approval no: 64.2018.mar). Rats were supplied by the Marmara University Experimental Animals Research and Implementation Centre (DEHAMER).
In total 32 Wistar albino male rats, weighing 250 – 300 g, aged 12 – 14 weeks were used in this study. During the experimental study, the rats were kept in cages (60 × 40 × 40 cm) with polyacrylic material and 4 rats were housed in each cage. Before the behavioural study, the rats were habituated with the reverse of the circadian cycle for 1 week (12-h:12-h light-dark cycles) where they were housed in rooms with a temperature of 22 ± 2°C, and humidity of 50 ± 5% with food and water ad libitum.
Drugs and chemicals
Vrx was purchased from (Lundbeck, Turkey). Vrx was freshly dissolved in distilled water before application (10 mg/kg). The dose and duration of Vrx treatment were based on previous studies found to be effective in stress and depression in rats (36, 37, 42). All drugs were administered by gavage for 7 days at the same time. All chemicals were supplied by Sigma (Sigma-Aldrich, St. Louis, MO, USA). All antibodies for immunoblotting were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA).
Experimental design and stress protocol
After the habituation period, the rats were divided randomly into four experimental groups each containing eight rats (n = 32, totally). These were (i) control: physiological saline solution (SS) (1 ml/kg/p.o.) (ii) Vrx (10 mg/kg/p.o.), (iii) Stress, and (i.v.) Stress + Vrx (10 mg/kg/p.o.). All treatments were given daily at the same time of the day (at 09.00 a.m.) for 7 days (Fig. 1).
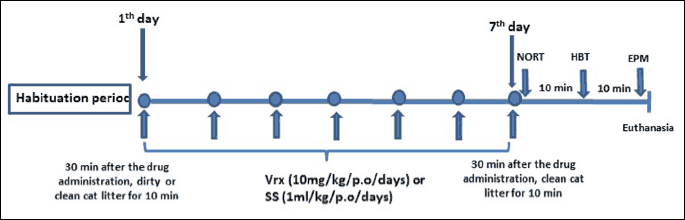
Abbreviations: EPM, elevated plus maze; HBT, hole board test; NORT, novel object recognition test; SS, saline solution; Vrx, vortioxetine.
The dirty cat litter, used by the same male cat for two days, was used as a stressor (24, 25). On the first day of the experiment, 1 hour after Vrx or saline treatment, the stress groups were exposed to dirty cat litter, while control and Vrx groups were exposed to clean (fresh and unused) cat litter. In the stress protocol, each rat was kept in a cage (30 cm × 30 cm × 40 cm) including 125 ml of dirty cat litter for 10 min unlike the unstressed group, which used the same amount of clean cat litter during the same period. Clean cat litter, as a situational reminder, was used in all groups before the behavioural experiments on the last day of the experiment (13, 22, 42).
Behavioural and cognition tests
Three different tests; NORT, HBT, and EPM tests were used for evaluation of cognitive and anxiety-like behaviour in rats. The EPM and HBT, extensively used to evaluate the fear or anxiety, have been validated for use in rats (43, 44). At the 7 day of experiments, 30 minutes after the last drug administration, the rats were exposed to clean cat litter for 10 min, and then immediately the rats were evaluated at NORT (43, 44), HBT (45), and EPM (13, 22, 42, 46); respectively (Fig. 1).
Novel object recognition test
Immediately after 10 min of clean cat litter application, NORT was performed to assess the object recognition memory functions of rats. Hippocampus is important for object recognition memory and hippocampal lesions produced moderate and reliable memory impairment (47). NORT is based on the principle that rodents will spend more time exploring a new object than the familiar one. In the present study, the object recognition tasks were applied to examine the short-term recognition memory using a 1-hour delay time. A black plexiglass box (50 cm × 50 cm × 30 cm) was used for the test. NORT has three steps as habituation, familiarizing and a test (44). Each rat has remained in the box without any objects for 10 minutes for habituation. Afterward, through the familiarization phase, a single animal is located for 5 min in the box comprising two similar sample objects on opposite sides of the box. After 1-hour delay time, during the test step, the rat is put back to the box with two objects, which one is similar to the sample and the other is novel and was allowed to explore objects for 3 min. The exposure time to the sample and to the novel object was recorded by a camera (43, 44). Object recognition was distinguished by more time spent interacting with the novel object and this was given a positive difference score. The difference score (A (s)) was calculated by the formula below (time spent with the novel object (B) and sample object (C)) (44, 45).
A= B – C
Hole board test
After 10 min of the NORT, the HBT was used to evaluate anxiety, exploration, and activity of the rats in an unfamiliar environment. The HBT performed in an apparatus with regularly arranged equally holes each of 3.8 cm in diameter on the floor (40 cm × 40 cm). The HBT was achieved by placing the rat in the centre of the board and recorded by a camera for 5 min. The hole-board test was performed by placing the rat in the centre of the board, and the test was recorded by a video camera for 5 min. When no movement of the head, trunk or extremities was observed, it was evaluated as cumulative freezing time. Increased freezing time indicates an elevation in anxiety (45, 48). Rearing behaviour is defined as the rat is stationary on its back paws and raises it is forepaws off the ground, extending its body vertically. Increased rearing frequency indicates that increased activity (49). Head dipping behaviour is defined as the rat places it’s head into one of the holes, to a minimum depth such that the ears were level with the floor of the apparatus. Increase head dipping indicates that increased exploratory tendency (49).
Elevated plus maze
Ten min after HBT, EPM was performed to evaluate anxiety-like behaviour. The wooden maze had four opposing arms, two open (50 cm × 10 cm) and two closed (50 cm × 10 cm) and was elevated 50 cm from the ground. The circumference of the two closed arms was surrounded by side and end walls 50 cm high. The open arms had no walls. Each rat was placed in the centre of the maze facing toward the open arms. On the last day of behavioural experiments, behaviours of the rats on EPM were recorded using a video camera for 5 min (= 300 s) (50). The cut-off criteria were that 5 minutes spent in closed arms and no entries into the open-arms on the EPM. Before placing each rat, all arms were cleaned with 70% ethanol. For each rat, it was recorded the number of entries in open arms (i), total number of entries into open and closed arms (ii), and time spent in open arms (iii). The anxiety index was calculated (13, 42) for each rat by the following formula:
Tissue collections
The rats in all the groups were decapitated under anesthetized with thiopental sodium (50 mg/kg) immediately after EPM. The brain tissues were collected from all animals and stored at –80°C for immunoblotting analysis. After decapitation, brain tissues were dissected as previously described (12, 50). The slides in the anteroposterior planes located between 7.20 – 5.70 mm, 6.70 – 4.70 mm, and 13.20 – 11.20 mm anterior to the interaural line were taken for the separation of the amygdaloid complex, the hippocampus, and the frontal cortex, respectively. The distance between lambda and bregma was accepted as 9.00 mm, posterior to the tip of the brain (51).
Immunoblotting assay
Frozen tissues were homogenized with a 20 mM Tris-HCl buffer co ntaining the protein inhibitor cocktail and then centrifuged at 2000 × g for 10 min. The obtained pellets, following centrifuge, were incubated with 0.5 mM DTT, 1% glycerol, 0.1 mM EDTA, 10 mM Tris-HCl, protease inhibitors and 0.05% Triton X-100 for 60 min. Different brain tissues were used according to the Lowry method (52) in the determination of the protein amount.
For the amygdala, the frontal cortex and the hippocampus, the prepared samples containing different concentrations of protein (50 µg, 100 µg, and 100 µg; respectively) were loaded on gel electrophoresis and transferred onto nitrocellulose membranes at 90 V for 60 min. Following the incubation overnight with primary antibodies [casp-3 (1:200) (cat. no: sc-56053), casp-9 (1:200) (cat. no: sc-56076), bcl-2 (1:200) (cat. no: sc-7382), bax (1:100) (cat. no: sc-20067), or precursor and mature BDNF (1:200) (cat. no: sc-546)] at +4°C, all the membranes were incubated with alkaline phosphatase-conjugated rabbit monoclonal anti-goat IgG secondary antibodies (1:1000) for 1 hour. Bio-Rad Molecular Analyst software (free edition, www.totallab.com) was used for densitometric analysis of the membranes. The molecular weights for casp-3, casp-9, bcl-2, bax, BDNF and b-actin (cat. no: sc-130657) (were used for standardization in all membranes) are 20, 46, 27, 23, 14/32, and 43 kDa; respectively.
Histologic analysis
The brain tissues were fixed by immersion in 10% neutral buffered formalin, embedded in paraffin as a standard procedure for light microscopy studies. Coronal brain sections -5 µm thick- were cut by a microtome, mounted on glass slides. Ten successive sections were selected by random systemic sampling from each animal and stained by Cresyl Violet for histopathological examinations. Slides were examined under a light microscope (Olympus CX41, Tokyo, Japan) linked to a digital camera (Kameram Dijital Mikroskopi, Turkey). Digital photographs were taken using a 10 × objective lens. The severity of the damaged neurons in the amygdaloid complex, frontal cortex, and dentate gyrus (DG) and Cornu ammonis regions CA1, CA2, CA3 of the hippocampus was scored semi-quantitatively by using a graded scale (0 to 3 score: 0 = no damage, 1 = mild damage, 2 = moderate damage, and 3 = severe damage). Degeneration of neurons was assessed as intensely stained nuclei, deep basophilia in cytoplasm, and shrinkage of the cell (14, 45, 53, 54). The average of the damage scores was calculated for each group (n = 8) and statistical analysis was performed.
Statistical analysis
For statistical analysis was used the GraphPad software (Prism 6.0; GraphPad Software, San Diego, CA, USA). All data were presented as mean ± SD. The statistical significance was determined by two-way analysis of variance (ANOVA), followed by Bonferroni multiple comparison post hoc tests. The value of P < 0.05 was considered to be statistically significant.
RESULTS
Novel object recognition test
According to NORT test results that were used to evaluate the impaired short-term memory function of the rats, the difference score was found to be significantly decreased in the stress group as compared to the control group (t = 4.031, P = 0.0039; dF = 28). The difference score was increased in the Stress + Vrx group compared to the stress group (t = 3.566, P = 0.0116; dF = 28; Fig. 2) and was not significantly changed in Vrx group as compared to the control group (P = n.s). It was increased by Vrx treatment respect to the stress group (t = 4.496, P = 0.0011; dF = 28; Fig. 2).
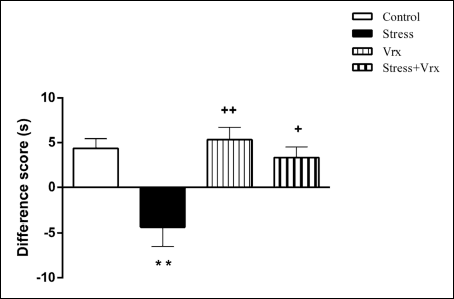 |
Fig. 2. According to NORT, difference score (s) the results of the control, stress, Vrx or Stress + Vrx groups. SS (1 ml/kg/p.o.) or Vrx (10 mg/kg/p.o.) treatments were administered for 7 days. On day 7 of treatment, all groups were exposed to clean cat litter (for 5 min), 30 minutes after treatment. NORT was applied to all groups after 10 min of clean litter application. **P < 0.01, compared to control group; +P < 0.05 and ++P < 0.01, compared to stress group. Each group consists of 8 rats. Abbreviations: NORT, novel object recognition test; SS, saline solution; Vrx, vortioxetine. |
Hole board test
HBT was used to assess anxiety-like behaviour of the rats. The frequency of rearing showing vertical activity was significantly diminished in the stress group compared to the control group (t = 4.295, P = 0.0021; dF = 28). Decreasing the rearing frequency was not significantly recovered by Vrx treatment in stress condition (t = 4.063, P = 0.0036; dF = 28). According to the test results, no effects were detected concerning this drug in Vrx group compared to the control group (p = n.s), unlike stress group (t = 6.269, P < 0.0001; dF = 28; Fig. 3A).
There was a significant increase in the cumulative freezing time showing anxiety in the stress group compared to the control and Vrx groups (t = 4.987, P = 0.0008; t = 4.302, P = 0.0033; dF = 28 in both groups), which was prevented by Vrx treatment (t = 3.544, P = 0.0162; dF = 28). Additionally, no effects were detected concerning this drug in Vrx group compared to the control group (Fig. 3B).
There was no difference in the number of head-dips scores showing exploratory behaviour between all groups (Fig. 3C).
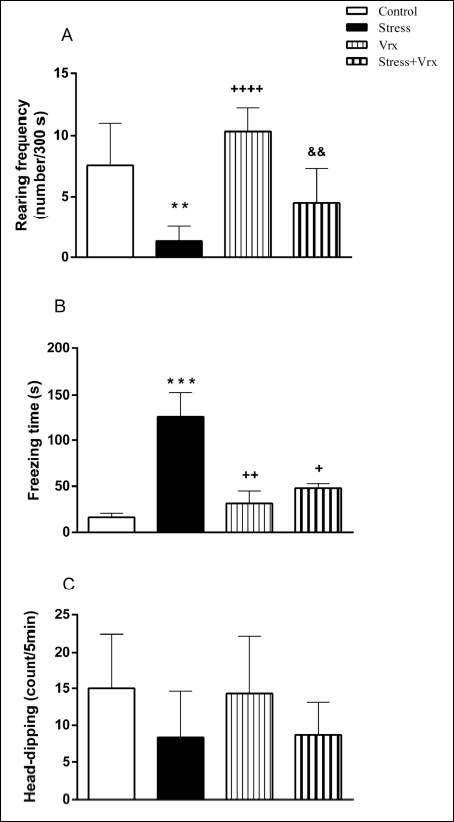 |
Fig. 3. Control, Stress, Vrx or Stress + Vrx groups rearing frequency (number/300 s) (A), cumulative freezing time (s) (B), head-dip (count/5 min) results obtained from HBT. SS (1 ml/kg/p.o.) or Vrx (10 mg/kg/p.o.) were administrated for 7 days. The HBT was applied to all groups 10 min after the NORT. **P < 0.01, ***P < 0.001, compared to control group; +P < 0.05, ++P < 0.01, ++++P < 0.0001, compared to stress group. &&P < 0.01 compared to Vrx group. Each group consists of 8 rats. Abbreviations: HBT, Hole board test; NORT, novel object recognition test; SS, saline solution; Vrx, vortioxetine. |
Elevated plus maze
When anxiety-like behaviours were evaluated by EPM, anxiety index (Nanxiety) of stress group was found to be significantly higher than the control group (t = 4.401, P = 0.0017; dF = 28; Fig. 4A). Vrx treatment significantly altered the anxiety index in the Stress + Vrx group (t = 5.948, P < 0.0001). The anxiety index was not detected differently in the Vrx group when compared with the control group (Fig. 4A).
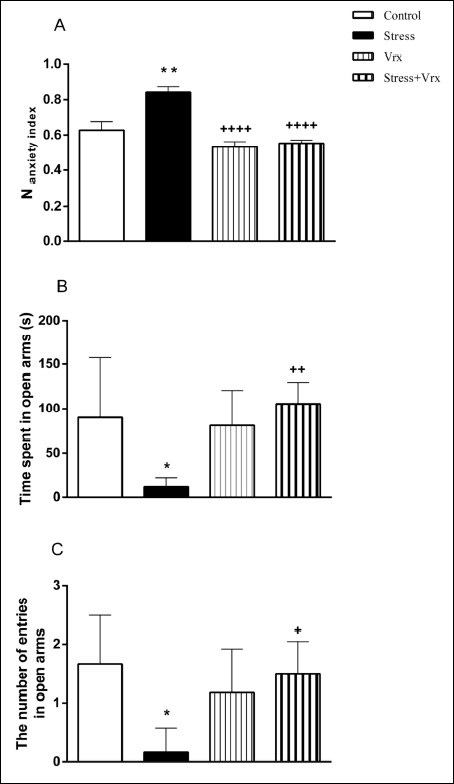 |
Fig. 4. The results of EPM: N anxiety index (A), time spent (s) (B), the number of entries in open arms (C). The EPM was applied to all groups 10 min after the HBT. *P < 0.05, **P < 0.01, compared to control group; +P < 0.05, ++P < 0.01, ++++P < 0.0001, compared to stress group. Each group consists of 8 rats. Abbreviations: EPM, elevated plus maze; HBT, Hole board test; Vrx, vortioxetine (10 mg/kg/p.o.). |
It was determined that the time spent in open arms decreased in the stress group compared to the control group (t = 3.237, P = 0.0248; dF = 28; Fig. 4B). In additionally, the decrease in the stress group was almost returned to control values with Vrx treatment (t = 3.900, P = 0.0053; dF = 28).
In the stress group, the number of entries in open arms decreased compared to the control (t = 3.985, P = 0.0044; dF = 28; Fig. 4C), but this reduction in the stress group was determined to ameliorate with the Vrx treatment (t = 3.542, P = 0.0123; Fig. 4C). The effect of Vrx treatment alone on the time spent in open arms and the number of entries in open arms was not determined (P: n.s; Fig. 4B and 4C).
The densitometric analysis of protein immunoblots
The representative immunoblotting membranes of the different brain tissues were illustrated in Fig. 5. The alterations of the bcl-2/bax expression ratio and caspase-3 and caspase-9 levels were used to determine the apoptosis evaluation in the mitochondrial apoptotic pathway. The ratio of anti-apoptotic (bcl-2) to pro-apoptotic proteins (bax) was calculated for each rat and each group (n = 4). On the other hand, BDNF contributes to the proliferation and the survival of central nervous system neurons, therefore BDNF levels were used to evaluate the brain neuroplasticity.
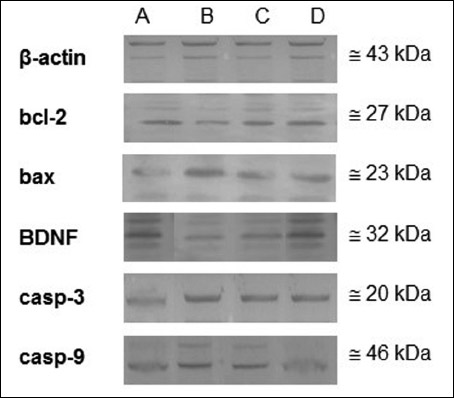 |
Fig. 5. The photographs of membranes collected from Western blotting experiments of Control (A), Stress (B), Vrx (C), or Stress + Vrx (D) in different brain regions. The immunoblots indicate the bcl-2, bax, casp-3, casp-9, and BDNF respectively (n = 4). β-actin was used to normalize the amount of protein loaded in each lane. Abbreviations: bax, bcl-2-associated X protein; bcl-2, b-cell lymphoma; BDNF, brain derived neurotrophic factor; casp-3, caspase-3; casp-9, caspase-9; Vrx: vortioxetine (10 mg/kg/p.o.). |
The expression of the bcl-2/bax ratio and BDNF were decreased in the stress group compared to the control group in the amygdaloid complex (t = 5.045, P = 0.0017; t = 4.087, P = 0.0090; dF = 12, respectively; Table 1). Caspase-3 and -9 levels were also significantly increased in the stress group compared to the control group in the amygdaloid complex (t = 4.561, P = 0.0039; t = 7.649, P < 0.0001; dF = 12, respectively). Vrx treatment exerted an increasing effect on bcl-2/bax ratio (t = 4.036, P = 0.0099, dF = 12), BDNF expression levels (t = 5.804, P = 0.0005, dF = 12) and decreasing effect on casp-3 (t = 3.333, P = 0.0358; dF = 12) and caspase-9 (t = 0.0002, P = 6.276; dF = 12) levels in the stress condition (Table 1).
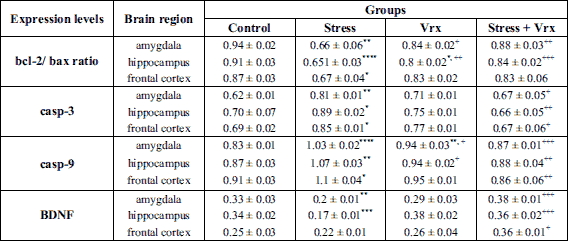
Abbreviations: bax, Bcl-2-associated X protein; bcl-2, b-cell lymphoma; BDNF, brain derived neurotrophic factor; casp-3, caspase-3; casp-9, caspase-9; PSS, predator scent stress; PTSD, post-traumatic stress disorder; SS, saline solution (1 ml/kg/p.o.); Vrx: vortioxetine (10 mg/kg/p.o.).
When the stress and control groups were compared for the hippocampal region, it was determined that bcl-2/bax ratio (t = 8.492, P < 0.0001; dF = 12) and BDNF levels (t = 6.098, P = 0.0003; dF = 12) decreased and casp-3 (t = 3.715, P = 0.0177; dF = 12) and casp-9 levels (t = 4.962, P = 0.002; dF = 12) increased (Table 1). The changes in the current protein expression levels in the stress condition were restored with Vrx treatment and it was found that almost returned to the control levels (t = 6.042, P = 0.0003 for bcl-2/bax ratio; t = 6.695, P = 0.0001 for BDNF level; t = 4.448, P = 0.0048 for casp-3; and t = 4.663, P = 0.0033, for casp-9).
When the stress group was compared to the control group, it was found that the expressions of bcl-2/bax ratio have reduced in the frontal cortex (t = 3.538, P = 0.0245; dF = 12), whereas the levels of casp-3 (t = 3.351, P = 0.0346; dF = 12) and casp-9 (t = 3.532, P = 0.0248; dF = 12) have increased (Table 1). In addition to all these findings, there was no change in BDNF expression level when the results of the study were compared between the control and the stress groups (P = n.s.). The increased casp-3 and casp-9 expression levels of stressed rats were improved with Vrx treatment (t = 3.666, P = 0.0194, dF = 12; t = 4.337, P = 0.0058, dF = 12). Also, BDNF expression level was increased with Vrx treatment in stress condition (t = 3.967, P = 0.0112, dF = 12). On the other hand, it was determined that the bcl-2/bax ratio has not changed with Vrx treatment compared to the stress group (P = n.s; Table 1). The effect of Vrx in the non-stressed rats was not detected in the frontal cortex.
Histopathologic analysis
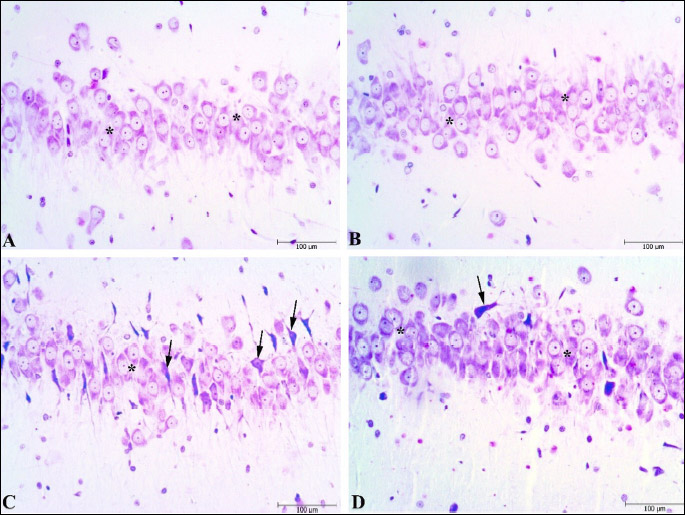
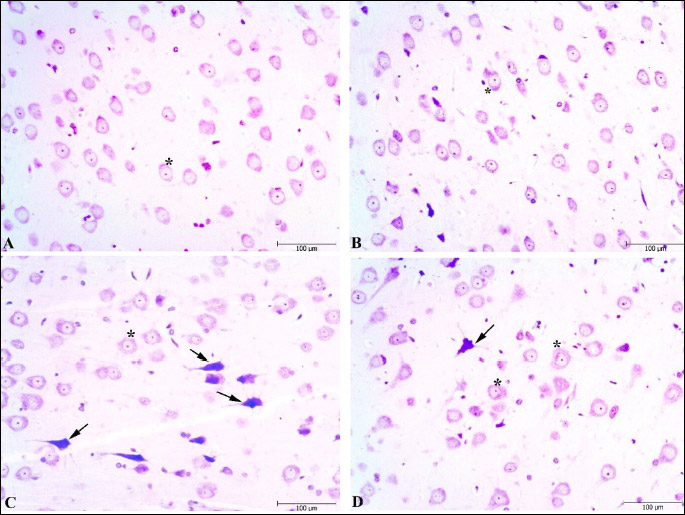
Histopathological scoring was performed to the effect of the Vrx treatment on the stress-related cellular damage in the amygdaloid complex, hippocampus, and frontal cortex regions (Fig. 6A-6C). As shown in Cresyl violet, the staining of the sections displayed cells with normal histomorphology in the control (Figs. 7A, 8A and 9A) and Vrx (Figs. 7B, 8B and 9B) groups. There were observed elevated damage scores in the stress group compared to the control group in the amygdaloid complex (t = 6.414, P < 0.0001; dF:28, Figs. 6A and 7C), hippocampal CA3 (t = 5.692, P = 0.0003; dF:28, Figs. 6B and 8C), and frontal cortex (t = 7.655, P < 0.0001; dF:28, Figs. 6C and 9C). The increased damage scores with PSS were partially recovered with Vrx treatment in the amygdaloid complex (t = 3.499, P = 0.0165; dF:28, Figs. 6A and 7D), the hippocampal CA3 (t = 3.111, P = 0.046; dF:28, Figs. 6B and 8D), and the frontal cortex (t = 3.661, P = 0.0107; dF:28, Figs. 6C and 9D). In the other hippocampal region there were no significant damage scores.
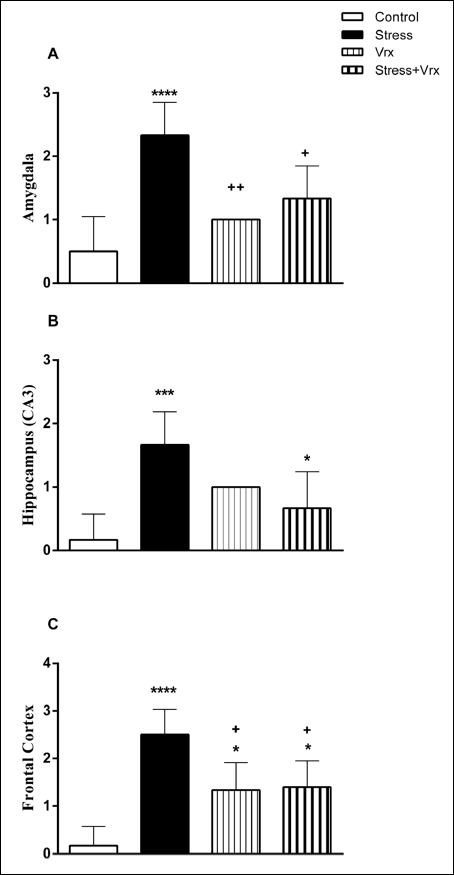 |
Fig. 6. Histopathological scores in the amygdala (A), hippocampus (B), frontal cortex (C) regions of rats. *P < 0.05, ***P < 0.001, ****P < 0.0001; compared to control and +P < 0.05, ++P < 0.01, compared to stress. Each group consists of 8 rats. |
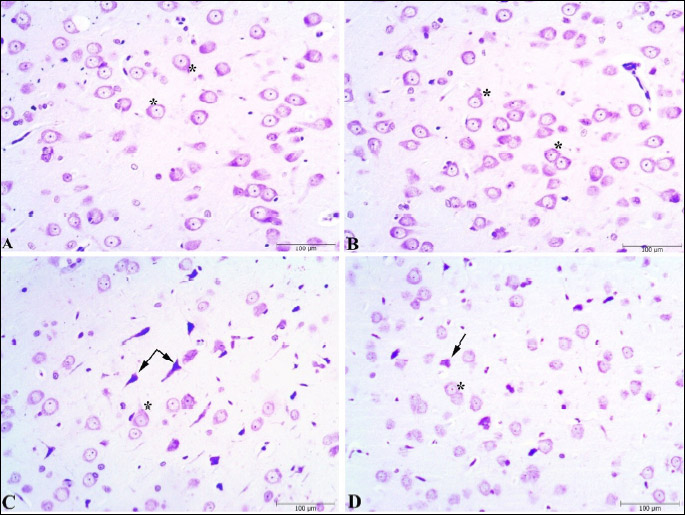
Vrx, vortioxetine (10 mg/kg/p.o.).
Vrx, vortioxetine (10 mg/kg/p.o.).
Vrx, vortioxetine (10 mg/kg/p.o.).
DISCUSSION
The major findings of the present study demonstrated that Vrx treatment improves behavioural and memory impairment in PSS rats. Stress-induced increased apoptotic parameters; bcl-2/bax ratio, casp-3 and -9 were decreased by Vrx treatments in the amygdaloid complex, the hippocampus, and the frontal cortex except for bcl-2/bax ratio. BDNF levels of all examined brain regions were increased with Vrx treatments in stress conditions. Also, histological damage is reduced by Vrx treatment in stress-induced rats.
We have found that PSS rats showed increased anxiety in both the EPM and HBT, while these increased anxiety-like behaviours were reversed with Vrx treatment. Previous studies have shown PSS, had no effect on head dipping (55), decreased head dipping (56), that showing exploratory activity in the hole board test. In agreements with these studies our study indicated head dipping was not affected with PSS and also Vrx did not change this exploratory behaviour. On the other hand, Adamec et al., have reported that predator stress, depressed ‘time active’ in HBT (57). In our study rearing frequency showing that vertical activity was diminished with PSS. Freezing time is also a commonly used behavioural parameter for evaluating anxiety in previous studies. Studies have reported increased freezing time (58, 59) and steady freezing time (60) in different maze test in PSS. Also, our EPM results support previous studies showing an increasing freezing time in PSS (14, 59, 61, 62). On the other hand, increased anxiety-like behaviours were prevented with Vrx treatment. Previously reported that acute administration of Vrx exerts anxiolytic-like effect (32, 63). Some human studies suggest that Vrx may have potential as a treatment option for generalized anxiety (40), social anxiety disorder (41), anxiety symptoms in persons with major depressive disorder (64). Our results showed that Vrx had an effect on anxiety. The mechanism of action of Vrx is complex, involving modulation of 5-HT receptors and SERT activity, resulting in modulation of several neurotransmitter systems. 5-HT3 receptor blockade and 5-HT1A receptor activation may be involved in anxiolytic effects (63).
Most of the studies of PTSD have found out impairments on attention/working memory, executive functions, retrospective, memory (24, 64). Working memory requires the proper functioning of the medial prefrontal cortex and hippocampus (28). Previously reported that predator scent cause impairment short-term working memory (24) which is consistent with the results of the current study. Additionally, patients with PTSD have been shown weakened performance in tests involving prefrontal lobe functions (65). In studies investigating cognitive dysfunction in PTSD patients, it has been shown that different cognitive functions have improved with SSRIs treatment (66, 67). Previously it was suggested that Vrx had acute therapeutic-like effects on cognitive memory and depression-like behaviours, and these effects were mediated by 5-HT receptors (68). Experimental studies suggest that 5-HT3 and 5-HT7 receptor antagonism, as well as 5-HT1A receptor agonism, may have a positive effect on cognitive functions including memory. Therefore, Vrx may potentially enhance memory (33). Wallace et al., demonstrated that Vrx restored impaired reversal learning via 5-HT depletion (36). Bertry et al., have shown Vrx increased the time spent with the novel object in the NORT (37). Similarly, our results showed that the recognition memory deficit induced by the PSS model is improved with Vrx treatment. This indicates that Vrx causes an ameliorative effect on hippocampal and PFC functions. Also, Vrx has been reported to contribute to anxiolysis by positively affecting the cognitive function (69, 70). Our results have shown that the cognitive function deficit in PSS model has been recovered by Vrx treatment. In accordance with the literature, the improvement of the deteriorated cognitive function may have an anxiolytic effect as shown in our study. Previous findings indicated that networks related to synaptic transmission, signal transduction, and neurodevelopment are modulated with Vrx and may underlie Vrx’s cognitive-enhancing properties (71). Additionally, blocking 5HT3 receptors by Vrx enhances the release of serotonin, norepinephrine, and acetylcholine which may be linked to pro-cognitive properties (70).
BDNF plays a critical role in synaptic plasticity (72). The dysfunction of synaptic plasticity was reported in PTSD (66). According to the neurotrophic hypothesis of mental disorders including stress, there is a down-regulation of BDNF in the different brain regions (72, 73). Similarly, physical stress also causes a decrease in systemic BDNF levels as central stress mediators in human subjects (74). On the other hand, it has been suggested that increasing BDNF levels led to decreased anxiety-like behaviours in rats (75, 76). In the present study, the decreased levels of the BDNF hippocampus due to PSS were increased by Vrx treatment, which may indicate increased neuroplasticity with Vrx treatment. Several studies have reported that BDNF levels significantly increased after 1 week with acute Vrx treatment in CA1 and DG (77). In light of previous studies, acute treatment was applied for a week to see the protective effect of the drug in a process that began with stress in the current study (77). Therefore, modulation of the neuroinflammation process along with neuroprotective agents may be useful for the treatment of mental disorders by increasing BDNF levels in PTSD patients (73). There are few studies showing the relationship between BDNF and stress-induced disorders including PSS rat models after antidepressant treatment (62,78,79). Lu et al., demonstrated that Vrx increased the hippocampal BDNF level in chronic unpredictable mild stressed animals (72). Furthermore, it has been demonstrated that Vrx regulated the expression of genes associated with the plasticity in the amygdala, the hippocampus, and the frontal cortex in rodents (80). In our study, increasing expression of BDNF in three different brain regions suggested that Vrx may stimulate neuroplasticity in stress conditions. In the present study, if there is no stress exposure, Vrx did not change the BDNF levels. However, stress-induced rats have increased BDNF levels. This suggests that Vrx may be effective in stressed conditions.
On the other hand, different PTSD models extensively suggest that an increase of BDNF in the amygdala (20, 62) which plays a key role in synaptic plasticity and the formation of fear memories. However, other studies reported that decreased BDNF levels in the amygdala (17, 18-20). Stress-induced epigenetic changes cause resulting in either transcriptional gene activation or repression of various genes, including BDNF (17). Our results show that a decreased in BDNF levels in the amygdala in the stress group. These discrepancies in literature may depend on the type of stress applied or the strain of the animal used.
Recent studies have shown that PTSD cause to increase in apoptosis in different brain regions, including the medial prefrontal cortex, the hippocampus, and the amygdala, which is associated with cognition and emotion (8-12). Apoptosis is thought to alter the volume and function in certain brain regions. The networks of γ-aminobutyric acid-ergic (GABAergic) interneurons in the amygdala are key factors of the brain’s inhibitory paths (6). GABA is essential for balancing between neuronal excitation and inhibition. Amygdala contains both glutamatergic and GABAergic interneurons (6, 81). Impairment of GABAergic inhibition in the amygdala can lead to behavioural hyperexcitability, such as increased anxiety and emotional dysregulation (82, 83). Stress may cause a reduction of the GABAergic inter-neuronal network and the development of neuropsychological diseases (83, 84). Our findings are consistent with previous data showing the crucial role of GABA innervation in the amygdala in fear condition. Skorzewska et al., have shown that fear conditioning reduces the GABA levels in the BLA by decreasing the mRNA level of the GABA synthesizing enzyme GAD67 in the amygdala and this condition leads to augmented fear reaction (83). Our immunoblotting results indicated that increased apoptosis in the amygdala in the stress group. This neuronal apoptosis is may related to GABAergic neurons and this leads to diminishing inhibition effect in the amygdala. Additionally, Zhao et al., have reported that predatory stress induces hippocampal cell death by apoptosis in rats (85). In agreement with this study our results indicated that PSS cause increased apoptosis in the hippocampus. On the other hand, previously it has been reported that in contrast to the significant atrophy in the hippocampal regions, amygdala displays increase dendritic arborization and spine density with PSS (16). In the present study we did not investigate dendritic hypertrophy or volume in amygdaloid complex.
Although there is no data about the anti-apoptotic effects of Vrx in PSS rat model, it has been shown that SSRIs were found to have beneficial effects in animal stress models (31, 86-88). SSRIs inhibit apoptosis on hippocampus by increasing BDNF level in the depression model (88). According to our immunoblotting results in the stressed group, the increase in pro-apoptotic proteins casp-3, casp-9, and bax levels and the decrease in anti-apoptotic bcl-2 level in three different regions emphasize the importance of apoptosis in cognitive disorders. Moreover, the suppression of apoptosis by Vrx treatment suggests that Vrx has a protective effect against stress disorders. In our study, the results of the histological damage assessments have shown that the increase in the neuronal damage caused by stress was partially reversed by Vrx treatment.
This study has focused on the acute effects of Vrx. Further studies are necessary in order to the long-term effects of Vrx in chronic PTSD models.
In conclusion, our results indicate that PSS causes anxiety and short-term recognition memory impairment and leads to apoptosis, and decreased BDNF levels in mainly brain regions. Neuronal deterioration is also accompanied by these findings. If administered right after trauma, Vrx treatment may significantly reduce behavioural, cognitive, and neuronal impairment increases neuroplasticity and may be protective against PTSD development.
Abbreviations: bax, bcl-2-associated X protein; BCIP, 5-bromo-4-chloro-3-indolyl phosphate; bcl-2, b-cell lymphoma; BLA, basolateral amygdala; BDNF, brain-derived neurotrophic factor; BSA, bovine serum albumin; CA, cornu ammonis; casp-3, caspase-3; casp-9, caspase-9; DG, dentate gyrus; EDTA, ethylenediaminetetraacetic acid; EPM, elevated plus maze; GABA, gama amino butiric acid; HBT, hole board test; i.v., intravenously kDa: kilodaltons; MeA, medial amygdala; NBT, nitro blue tetrazolium; NORT, novel object recognition test; PFC, prefrontal cortex; PMSF, phenylmethanesulfonyl fluoride; p.o., perorally; PSS, predator scent-stress; SS, saline solution; PTSD, post-traumatic stress disorder; SD, standard deviation; SSRI, selective serotonin reuptake inhibitor; TBS, Tris buffer saline; TBS-T, Tris buffer saline 0.05% Tween-20; Vrx, vortioxetine.
This research did not receive any specific grant from funding agencies in the public, the commercial, or the not-for-profit sectors.
Conflict of interests: None declared.
REFERENCES
- Girgenti MJ, Hare BD, Ghosal S, Duman RS. Molecular and cellular effects of traumatic stress: implications for PTSD. Curr Psychiatry Rep 2017; 19: 85. doi: 10.1007/s11920-017-0841-3
- Schoenfeld TJ, Rhee D, Martin L, Smith S, Sonti A, Padmanaban VS. New neurons restore structural and behavioural abnormalities in a rat model of PTSD. Hippocampus 2019; 29: 848-861.
- Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry 2015; 206: 93-100.
- Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD) J Affect Disord 2002; 70:1-17. doi: 10.1016/s0165-0327(01)00351-2
- Zhang LM, Zhang YZ, Li YF. The progress of neurobiological mechanisms on PTSD. Chin Pharmacol Bull 2010; 26: 704-707.
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164: 1476-1488.
- Chis IC, Clichici A, Nagy AL, Oros A, Catoi C, Clichici S. Quercetin in association with moderate exercise training attenuates injuries induced by experimental diabetes in sciatic nerves. J Physiol Pharmacol 2017; 68: 877-886.
- Jia Y, Han Y, Wang X, Han F. Role of apoptosis in the post-traumatic stress disorder model-single prolonged stressed rats. Psychoneuroendocrinology 2018; 95: 97-105.
- Xiao B, Yu B, Wang HT, Han F, Shi YX. Single-prolonged stress induces apoptosis by activating cytochrome C/caspase-9 pathway in a rat model of post-traumatic stress disorder. Cell Mol Neurobiol 2011; 31: 37-43.
- Ding J, Han F, Shi Y. Single-prolonged stress induces apoptosis in the amygdala in a rat model of post-traumatic stress disorder. J Psychiatr Res 2010; 44: 48-55.
- Kuhn HG, Palmer TD, Fuchs E. Adult neurogenesis: a compensatory mechanism for neuronal damage. Eur Arch Psychiatry Clin Neurosci 2001; 251: 152-158.
- Du J, Wang Y, Hunter R, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci USA 2009; 106: 3543-3548.
- Aykac A, Aydyn B, Cabadak H, Goren MZ. The change in muscarinic receptor subtypes in different brain regions of rats treated with fluoxetine or propranolol in a post-traumatic stress disorder model. Behav Brain Res 2012; 232: 124-129.
- Ozbeyli D, Gokalp AG, Koral T, et al. Protective effect of exercise and sildenafil on acute stress and cognitive function. Physiol Behav 2015; 151: 230-237.
- Bremmer DJ. Traumatic stress: effects on the brain. Dialogues Clin Neurosci 2006; 8: 445-461.
- Cohen H, Kozlovsky N, Matar MA, Zohar J, Kaplan Z. Distinctive hippocampal and amygdalar cytoarchitectural changes underlie specific patterns of behavioral disruption following stress exposure in an animal model of PTSD. Eur Neuropsychopharmacol 2014; 24: 1925-1944.
- Solanki N, Alkadhi I, Atrooz F, Patki G, Salim S. Grape powder prevents cognitive, behavioral, and biochemical impairments in a rat model of posttraumatic stress disorder. Nutr Res 2015; 35: 65-75.
- Li G, Wang G, Shi J, et al. Trans-resveratrol ameliorates anxiety-like behaviors and fear memory deficits in a rat model of post-traumatic stress disorder. Neuropharmacology 2018; 133: 181-188.
- Asim M, Hao B, Yang YH, et al. Ketamine alleviates fear generalization through GluN2B-BDNF signaling in mice. Neurosci Bull 2019; Aug 23: doi: 10.1007/s12264-019-00422-47
- Ji LL, Ye Y, Nie PY, et al. Dysregulation of miR-142 results in anxiety-like behaviors following single prolonged stress. Behav Brain Res 2019; 365: 157-163.
- Zoladz PR, Park, CR, Halonen JD, et al. Differential expression of molecular markers of synaptic plasticity in the hippocampus, prefrontal cortex, and amygdala in response to spatial learning, predator exposure, and stress-induced amnesia. Hippocampus 2011; 22; 577-589.
- Cohen H, Matar MA, Joseph Z. Animal models of post-traumatic stress. Preclinical models of neurologic and psychiatric disorders. Curr Protoc Neurosci 2013; 9.45: doi: 10.1002/0471142301.ns0945s64.
- Adamec R, Bartoszyk GD, Burton P. Effects of systemic injections of vilazodone, a selective serotonin reuptake inhibitor and serotonin 1A receptor agonist, on anxiety induced by predator stress in rats. Eur J Pharmacol 2004; 504: 65-77.
- Morrow BA, Roth RH, Elsworth JD. TMT, a predator odor, elevates mesoprefrontal dopamine metabolic activity and disrupts short-term working memory in the rat. Brain Res Bull 2000; 52: 519-523.
- Shallcross J, Hamor P, Bechard AR, Romano M, Knackstedt L, Schwendt M. The divergent effects of CDPPB and cannabidiol on fear extinction and anxiety in a predator scent stress model of PTSD in rats. Front Behav Neurosci 2019; 13: 91. doi: 10.3389/fnbeh.2019.00091
- Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute episodes of predator exposure in conjunction with chronic social instability as an animal model of post-traumatic stress disorder. Stress 2008; 11: 259-281.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Arlington VA, American Psychiatric Publishing, 2013.
- Torrisi S, Leggio GM, Drago F, Salomone S. Therapeutic challenges of post-traumatic stress disorder: focus on the dopaminergic system. Front Pharmacol 2019; 10: 404. doi: 10.3389/fphar.2019.00404
- Davidson JR. Pharmacologic treatment of acute and chronic stress following trauma: J Clin Psychiatry 2006; 67: 34-39.
- Czeh B, Michaelis T, Watanabe T, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA 2001; 98: 12796-12801.
- Lucassen PJ, Fuchs E, Czeh B. Antidepressant treatment with tianeptine reduces apoptosis in the hippocampal dentate gyrus and temporal cortex. Biol Psychiatry 2004; 55: 789-796.
- Sowa-Kucma M, Panczyszyn-Trzewik P, Misztak P, et al. Vortioxetine: a review of the pharmacology and clinical profile of the novel antidepressant. Pharmacol Rep 2017; 69: 595-601.
- Mork A, Montezinho LP, Miller S, et al. Vortioxetine (Lu AA21004), a novel multimodal antidepressant, enhances memory in rats. Pharmacol Biochem Behav 2013; 105: 41-50.
- Sanchez C, Asin KE, Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther 2015; 145: 43-57.
- Pehrson AL, Hillhouse TM, Haddjeri N, et al. Task- and treatment length-dependent effects of vortioxetineon scopolamine-induced cognitive dysfunction and hippocampal extracellular acetylcholine in rats. J Pharmacol Exp Ther 2016; 358: 472-482.
- Wallace A, Pehrson AL, Sanchez C, Morilak DA. Vortioxetine restores reversal learning impaired by 5-HT depletion or chronic intermittent cold stress in rats. Int J Neuropsychopharmacol 2014; 17: 1695-1706.
- Betry C, Etievant A, Pehrson A, Sanchez C, Haddjeri N. Effect of the multimodal acting antidepressant vortioxetine on rat hippocampal plasticity and recognition memory. Prog Neuropsychopharmacol Biol Psychiatry 2015; 58: 38-46.
- Lu Y, Ho CS, McIntyre RS, Wang W, Ho RC. Effects of vortioxetine and fluoxetine on the level of brain derived neurotrophic factors (BDNF) in the hippocampus of chronic unpredictable mild stress-induced depressive rats. Brain Res Bull 2018; 142: 1-7. doi: 10.1016/j.brainresbull.2018.06.007
- Orsolini L, Tomasetti C, Valchera A, et al. New advances in the treatment of generalized anxiety disorder: the multimodal antidepressant vortioxetine. Expert Rev Neurother 2016; 16: 483-495.
- Pae CU, Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, Serretti A. Vortioxetine, a multimodal antidepressant for generalized anxiety disorder: a systematic review and meta-analysis. J Psychiatr Res 2015; 64: 88-98.
- Liebowitz MR, Careri J, Blatt K, et al. Vortioxetine versus placebo in major depressive disorder comorbid with social anxiety disorder. Depress Anxiety 2017; 34: 1164-1172.
- Pellow S, Chopin P, File SE, Briley M. Validation of open-closed arm entries in an elevated plus maze as measure of anxiety in the rat. J Neurosci Methods 1985; 14: 149-167.
- Ozbeyli D, Sari G, Ozkan N, et al. Protective effects of different exercise modalities in an Alzheimer’s disease-like model. Behav Brain Res 2017; 328: 159-177.
- Bevins R, Besheer J. Object recognition in rats and mice: a one-trial non-matching to sample learning task to study recognition memory. Nat Protoc 2006; 1: 1306-1311.
- Kasimay-Cakir O, Ellek N, Salehin N, et al. Protective effect of low dose caffeine on psychological stress and cognitive function. Physiol Behav 2017; 168: 1-10.
- Aykac A, Karanlik B. The expression level of muscarinic M1 receptor subtypes in different regions of rat brain. Marmara Med J 2017; 30: 162-168.
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem 2010; 17: 5-11.
- Kasimay O, Guzel E, Gemici A, et al. Colitis-induced oxidative damage of the colon and skeletal muscle is ameliorated by regular exercise in rats: the anxiolytic role of exercise. Exp Physiol 2006; 91: 897-906.
- Adamec R, Berton O, Razek WA. Viral vector induction of CREB expression in the periaqueductal gray induces a predator stress-like pattern of changes in pCREB expression, neuroplasticity, and anxiety in rodents. Neural Plast 2009; 2009: 904568. doi: 10.1155/2009/904568
- Cohen H, Kaplan Z, Matar MA, Loewenthal U, Kozlovsky N, Zohar J. Anisomycin, a protein synthesis inhibitor, disrupts traumatic memory consolidation and attenuates posttraumatic stress response in rats. Biol Psychiatry 2006; 60: 767-776.
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. London, Academic Press, 1986.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin-phenol reagents. J Biol Ther 1951; 191: 33-43.
- Koo E, Sheldon RA, Lee BS, Vexler ZS, Ferriero DM. Effects of therapeutic hypothermia on white matter injury from murine neonatal hypoxia-ischemia. Pediatr Res 2017; 82: 518-526.
- Koyuncuoglu T, Vyzdyklar C, Uren D, et al. Obestatin improves oxidative brain damage and memory dysfunction in rats induced with an epileptic seizure. Peptides 2017; 90: 37-47.
- Adamec R, Muir C, Grimes M, Pearcey K. Involvement of noradrenergic and corticoid receptors in the consolidation of the lasting anxiogenic effects of predator stress. Behav Brain Res 2007; 179: 192-207.
- Adamec R, Fougere D, Risbrough V. CRF receptor blockade prevents initiation and consolidation of stress effects on affect in the predator stress model of PTSD. Int J Neuropsychopharmacol 2010; 13: 747-757.
- Adamec R, Head D, Soreq H, Blundell J. The role of the read through variant of acetylcholinesterase in anxiogenic effects of predator stress in mice. Behav Brain Res 2008; 189: 180-190.
- Dopfel D, Perez PD, Verbitsky A, et al. Individual variability in behavior and functional networks predicts vulnerability using an animal model of PTSD. Nat Commun 2019; 10: 2372. doi: 10.1038/s41467-019-09926-z
- Hoffman JR, Ostfeld I, Kaplan Z, Zohar J, Cohen H. Exercise enhances the behavioral responses to acute stress in an animal model of PTSD. Med Sci Sports Exerc 2015; 47: 2043-2052.
- Saridogan GE, Aykac A, Cabadak H, Cerit C, Caliskan M, Goren MZ. d-Cycloserine acts via increasing the GluN1 protein expressions in the frontal cortex and decreases the avoidance and risk assessment behaviors in a rat traumatic stress model. Behav Brain Res 2015; 293: 227-233.
- Hoffman JR, Ostfeld I, Stout JR, Harris RC, Kaplan Z, Cohen H. b-alanine supplemented diets enhance behavioral resilience to stress exposure in an animal model of PTSD. Amino Acids 2015; 47: 1247-1257.
- Cohen H, Zohar J, Carmi L. Effects of agomelatine on behaviour, circadian expression of period 1 and period 2 clock genes and neuroplastic markers in the predator scent stress rat model of PTSD. World J Biol Psychiatry 2018; Nov 1: 1-19. doi: 10.1080/15622975.2018.1523560
- Guilloux JP, Mendez-David I, Pehrson A, et al. Antidepressant and anxiolytic potential of the multimodal antidepressant vortioxetine (Lu AA21004) assessed by behavioural and neurogenesis outcomes in mice. Neuropharmacology 2013; 73: 147-159.
- Scott JC, Woods SP, Wrocklage KM, Schweinsburg BC, Southwick SM, Krystal JH. Prospective memory in posttraumatic stress disorder. J Int Neuropsychol Soc 2016; 22: 724-734.
- Kocak EE, Kilic C. Cognitive dysfunctions in posttraumatic stress disorder. Turk Psikiyatri Derg 2017; 28: 1-7.
- Fani N, Kitayama N, Ashraf A, et al. Neuropsychological functioning in patients with posttraumatic stress disorder following short-term paroxetine treatment. Psychopharmacol Bull 2009; 42: 53-68.
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry 2003; 54: 693-702.
- du Jardin KG, Liebenberg N, Mueller HK, Elfving B, Sanchez C, Wegener G. Differential interaction with the serotonin system by S-ketamine, vortioxetine, and fluoxetine in a genetic rat model of depression. Psychopharmacology (Berl) 2016; 233: 2813-2825.
- McIntyre RS, Xiao HX, Syeda K, et al. The prevalence, measurement, and treatment of the cognitive dimension/domain in major depressive disorder. CNS Drugs 2015; 29: 577-589.
- Stahl SM. Modes and nodes explain the mechanism of action of vortioxetine, a multimodal agent (MMA): blocking 5HT3 receptors enhances release of serotonin, norepinephrine, and acetylcholine. CNS Spectrums 2015; 20: 455-459.
- Waller JA, Nygaard SH, Li Y, et al. Neuroplasticity pathways and protein-interaction networks are modulated by vortioxetine in rodents. BMC Neurosci 2017; 18: 56. doi: 10.1186/s12868-017-0376-x
- Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol 2014; 220: 223-250.
- Lee B, Hong R, Lim P, et al. The ethanolic extract of Aralia continentalis ameliorates cognitive deficits via modifications of BDNF expression and anti-inflammatory effects in a rat model of post-traumatic stress disorder. BMC Complement Altern Med 2019; 19: 9-11.
- Verbickas V, Baranauskiene N, Eimantas N, et al. Effect of sprint cycling and stretch-shortening cycle exercises on the neuromuscular, immune and stress indicators in young men. J Physiol Pharmacol 2017; 68: 125-132.
- Calabrese F, Molteni, R, Racagni G, Riva MA. Neuronal plasticity: a link between stress and mood disorders. Psychoneuroendocrinology 2009; 34: 208-216.
- Song X, Liu B, Cui L, et al. Silibinin ameliorates anxiety/depression-like behaviors in amyloid b-treated rats by upregulating BDNF/TrkB pathway and attenuating autophagy in hippocampus. Physiol Behav 2017; 179: 487-493.
- Chen F, Danladi J, Ardalan M, et al. A critical role of mitochondria in BDNF-associated synaptic plasticity after one-week vortioxetine treatment. Int J Neuropsychopharmacol 2018: 21: 603-615.
- Aykac A, Oncul S, Onur R. Social isolation and predator scent tests alter brain BDNF levels differentially according to gender, in rats and effects of fluoxetine. J Res Pharm 2018; 22: 190-198.
- Yu H, Chen JJ, Zeng BQ, Zhong QP, Xu JP, Liu YG. Role of cAMP/CREB/BDNF signaling pathway in anti-depressive effect of vortioxetine in mice. Nan Fang Yi Ke Da Xue Xue Bao 2017; 37: 107-112.
- Waller JA, Tamm JA, Abdourahman A, Pehrson AL, Li Y, Cajina M, Sanchez C. Chronic vortioxetine treatment in rodents modulates gene expression of neurodevelopmental and plasticity markers. Eur Neuropsychopharmacol 2017; 27: 192-203.
- Bhatnagar S, Vining C, Denski K. Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann NY Acad Sci 2004; 1032: 315-319.
- Prager EM, Bergstrom HC, Wynn GH, Braga MF. The basolateral amygdala g-aminobutyric acidergic system in health and disease. J Neurosci Res 2016; 94: 548-567.
- Skorzewska A, Wislowska-Stanek A, Lehner M, et al. Corticotropin releasing factor receptor 1 antagonist differentially inhibits freezing behavior and changes gamma-aminobutyric acidergic activity in the amygdala in low- and high-anxiety rats. J Physiol Pharmacol 2017; 68: 35-46.
- Jie F, Yin G, Yang W, et al. Stress in regulation of GABA amygdala system and relevance to neuropsychiatric diseases. Front Neurosci 2018; 12: 562. doi: 10.3389/fnins.2018.00562
- Zhao H, Xu H, Xu X, Young D. Predatory stress induces hippocampal cell death by apoptosis in rats. Neurosci Lett 2007; 421: 115-120.
- Aykac A, Cabadak H, Goren MZ. Altered ratio of proapoptotic and antiapoptotic proteins in different brain regions of female rats in model of post-traumatic stress disorder. Turk J Biochem 2015; 40: 1-7. doi: 10.5505/tjb.2015.50479
- Djordjevic A, Djordjevic J, Elakovic I, Adzic M, Matic G, Radojcic MB. Effects of fluoxetine on plasticity and apoptosis evoked by chronic stress in rat prefrontal cortex. Eur J Pharmacol 2000; 693: 37-44.
- Huang X, Mao YS, Li C, Wang H, Ji JL. Venlafaxine inhibits apoptosis of hippocampal neurons by up-regulating brain-derived neurotrophic factor in a rat depression model, Pharmazie 2014; 69: 909-916.
A c c e p t e d : August 28, 2019