The gastrointestinal tract and the immune system are particularly responsive to different stressors. In the past years the influence of psychosocial and environmental stressors on the pathogenesis of the gastrointestinal diseases has received increased awareness (2). Stress may affect different physiologic functions of the gastrointestinal tract including gastric secretion, gut motility, mucosal permeability and barrier function, visceral sensitivity and mucosal blood flow (3-5) (Fig. 1). In recent years the important interplay between stress and gut microbiota has been shown. Interestingly, bacteria may respond directly to stress-related host signals. There is evidence that catecholamines can alter the growth, motility and virulence of pathogenic and commensal bacteria. Thereby, stress may influence the outcome of infections by these bacteria in many hosts (6).
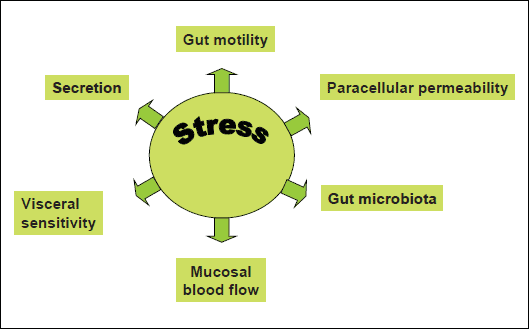 |
Fig. 1. Effect of stress on gastrointestinal functions. Stress has impact on important physiological functions of gut including gut motility, secretion, visceral sensitivity, mucosal blood flow. In addition, stress modifies gut microbiota and enhances paracellular permeability. |
This article reviews the impact of stress on the gastrointestinal tract. Especially the focus is addressed to the role of stress in the pathophysiology of the most common diseases of the gastrointestinal tract and to the diagnostic and therapeutic options to prevent stress-related disorders.
Concerning the link between stress and gastrointestinal diseases, most people are aware of the fact that the central nervous system and the gut are intimately connected. It is well known that exposure to stress may lead to the manifestation of different symptoms within the gastrointestinal tract such as dyspepsia, diarrhoea or abdominal pain. The first observation performed on the wounded soldier with gastric fistula by William Beaumont showed that fear or anger may significantly influence the gastric physiology, especially acid secretion (7). In the later years, the major breakthrough in understanding the interactions between the central nervous system (CNS) and the gut was the discovery of the enteric nervous system (ENS) in the nineteenth century. ENS (called also "little brain") plays a crucial role in the regulation of the physiological gut functions including secretion, motility and release of various neuropeptides and hormones (8). Brain communicates with the gut through multiple parallel pathways including autonomic nervous system (ANS), the hypothalamic pituitary-adrenal axis (HPA), and other connections, which were termed the brain-gut-axis (BGA) (Fig. 2) (9-10). Based on previous studies there is strong evidence that exposure to stress may be responsible for the dysregulation of the BGA, thus leading to the different diseases of the gut (11).
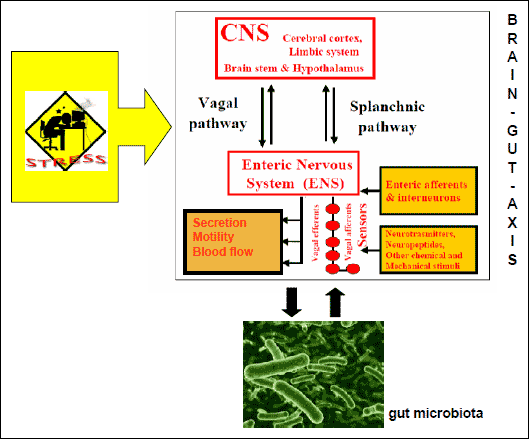 |
Fig. 2. Impact of stress on brain-gut-microbiota axis. There is a bidirectional interaction between brain-gut axis and gut microbiota. |
One of the important coordinators of the endocrine, behavioural and immune response to stress is corticotrophin releasing factor (CRF). The CRF family of peptides are expressed in the CNS and within the gut and displays potent biologic actions. CRF has a potent effects on gut via modulation of inflammation, increase of gut permeability, contribution to visceral hypersensitivity (increased perception to pain) and modulation of the gut motility. CRF release in the hypothalamus is the first step in activation of HPA involved in stress response. This represents the major endocrine response system to stress. The pituitary gland responds to CRF by releasing of adrenocorticotropic hormone (ACTH) to stimulate adrenal glands secretion of the stress hormone cortisol (12).
The topic of the brain-gut axis has recently received even more attention due to the discovery of the bidirectional interaction between the brain-gut axis and gut microbiota. The cross talk between gut microbiota, the immune system and the brain-gut axis plays an important role in the modulation of the stress response of the gut in the context of the development of different gut disorders (13).
There is also an evidence that gut bacteria helps to keep the bidirectional contact between the components of the brain and gut axis. In other words exposure to stress modifies the bacterial flora, but also the opposite is true that the gut bacteria, which may have a profound effect on the BGA and may modulate motility, permeability and visceral sensitivity. Microbiota communicate with the BGA through different mechanisms: 1) direct interaction with mucosal cells (endocrine message), 2) via immune cells (immune message) and finally 3) via contact to neural endings (neuronal message) (13).
Stress causes changes in the composition of the microbiota; induces changes in neurotransmitter and proinflammatory cytokine levels, which could affect directly or indirectly the microbiota. For example, norepinephrine increases the virulence of some, bacteria like E. coli or C. jejuni. Gut microbiota may modulate the sensation to pain and some probiotics may inhibit the hypersensitivity and perhaps the intestinal permeability caused by the body's exposure to stress. Multiple lines of evidence illustrate the impressive cross talk between stress, immune system and the gut microbiota (14).
Our own studies documented that a probiotic bacterial strain of E. coli Nissle (EcN) may significantly reduce the stress-induced gastric lesions, however this effect was attenuated, at least in part, due to the blockade of the sensory nerves with capsaicin. The role of sensory afferent nerves is supported by the fact that the addition of exogenous CGRP to rats with sensitive inactivated by capsaicin sensory nerves, the important neuromediator of these nerve endings restores the protective effect of probiotics. This observation supports a close link between the enteric nervous system (ENS) and microbiota in the mechanism of protection of the gastric mucosa (15).
Concerning the translation of the stress signals to the gut, mast cells play an important role. Interestingly, these cells secrete a number of important mediators and have on their surface the receptors for the CRF indicating an important link between stress and these cells (16, 17).
Finally, the exposure to chronic stress is associated with prolonged and excessive activation of stress response areas within the CNS. This exposure may cause even irreversible changes in the brain areas responsible for the perception of pain in the gut. These changes can be shown using so called functional magnetic resonance imaging (MRI) techniques (18).
A consequence of the dysregulation of BGA induced by exposure to stress may lead to the development of a broad array of gastrointestinal diseases such as gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), IBD, IBS and even food allergy (20, 21) (Fig. 3).
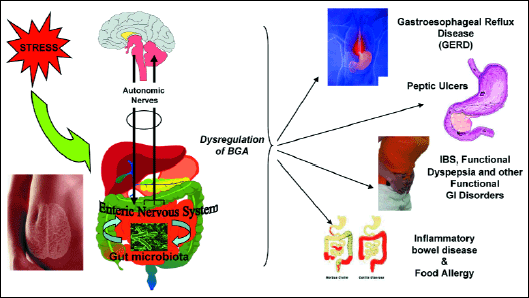 |
Fig. 3. Pathophysiological consequences of the disruption of brain-gut-microbiota axis by stress. The exposure to stress leads to disturbance of brain-gut axis (BGA) resulting in the development of different diseases of gastrointestinal tract including gastroesophageal reflux disease, peptic ulcer disease, irritable bowel disease, inflammatory bowel disease and food allergy. |
Gastroesophageal reflux disease (GERD) represents one of the most important manifestations of stress exposure to GI tract. It has been shown that stress causes the aggravation of GERD symptoms due to inhibition of the lower esophageal sphincter and increased sensitivity to acid i.e. an increased perception of acid refluxate. On the other side the reduction of stress may lead to an improvement of GERD symptoms (22). Interestingly, during stress exposure the amount of reflux does not always increases, but the probability of a feeling of reflux as heartburn increase. Generally, the treatment for GERD, especially in those who are not responsive to anti-reflux therapy (PPI), requires further evaluation of potential effect of stress on patients subjected to PPI therapy (23).
Concerning the link between stress and GERD, Li et al. (24) and Perlman et al. (25) have demonstrated in their recent studies the strong impact of acute stress such as terroristic attack on World Trade Center on GERD symptoms.
Exposure to a stressful life events may evoke the development of PUD. Ulcer patients are more likely to be divorced, separated or widowed. Despite the fact that Helicobacter pylori (Hp) and nonsteroidal anti-inflammatory drugs (NSAID) are the important causes of PUD, it has been shown that stress exposure may contribute to this disease and may impede the gastric and duodenal defences against the damage induced by an attack from acid and pepsin damage (26). Possible causative factors include: 1) alterations in gastric acid secretion; 2) reduced mucosal blood flow; 3) reduced HCO3- secretion; 4) acid back diffusion; 5) reduced proliferation and restitution of the injured mucosa; 6) alterations in gastric motility. Before the discovery of Hp, stress was considered as one of the most important risk factor for peptic ulceration (27). A special form of ulcerations resulting from intense stress exposure is termed as a stress ulceration, commonly observed in patients in intensive care units (28).
Our own studies demonstrated that stress may profoundly affect the BGA via the modulation of a number of important neuropeptides (like CGRP) involved in the protection of the gastric mucosa, changes in gastric secretion, regeneration of gastric mucosa, and changes in mucosal blood flow (29-30) (Fig. 4).
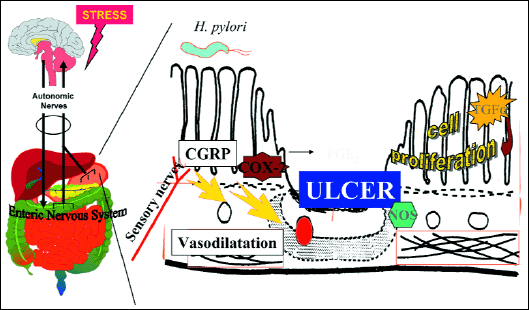 |
Fig. 4. Effect of disruption of brain-gut axis on ulcer healing. Exposure to stress and the resulting disturbance of brain-gut axis may have negative effect on ulcer healing including changes in gastric secretion, proliferation rate at the ulcer edge and angiogenesis. |
From a practical point of view, the diagnostic approach to stress-related diseases in the upper GI tract includes non-invasive and invasive techniques (31). Non-invasive techniques consist of 1) routine blood tests (complete blood count, chemistries, thyroid function, stool diagnostic); 2) celiac disease serology (transglutaminase IgA), 13C urease breath test to exclude Hp infection, determination of elastase-1 in stool to exclude exocrine pancreatic insufficiency, H2 breath test with glucose to exclude small intestinal bacterial overgrowth (SIBO), H2 breath testing to rule out carbohydrate malabsorption, abdominal ultrasonography and other imaging methods (MRI), sucrose permeability test to analyze the intestinal barrier function and evaluation of the gut motility with H2 breath test using lactulose. The invasive techniques include upper GI tract endoscopy or intestinal endoscopy (capsule endoscopy, push-and pull enteroscopy).
Therapies of stress-related disorders within the upper GI tract are focused mainly on the inhibition of gastric acid secretion using proton pump inhibitors (PPI), histamine-H2 blockers, etc. In the case of Hp infection, an appropriate eradication should be advocated, because the eradication of Hp may lead in some patients with functional dyspepsia and an improvement of the symptoms (32, 33). Recently, some studies indicate a positive effect of probiotics on stress related pathology in upper GI tract, however the effects need to be further evaluated (34).
Stress is also a known risk factor for the induction and exacerbation of IBD. There is evidence that stress may induce de novo colitis or provokes the exacerbation of colitis (35). However, not all studies support this association, so more large prospective population-based studies are needed to better explore these potential interaction. Additionally, animal models are another line of evidence (36). Numerous studies have demonstrated that stress may aggravate the experimental colitis by increasing oxidative damage (37). Finally, human studies revealed a close association between acute daily stressors and various bowel symptoms (38). The exact mechanisms by which stress exposure may induce or aggravate colitis is not well known, but probably by activation of mast cells, increased release of cells and impairment of the intestinal barrier function (20) (Fig. 5).
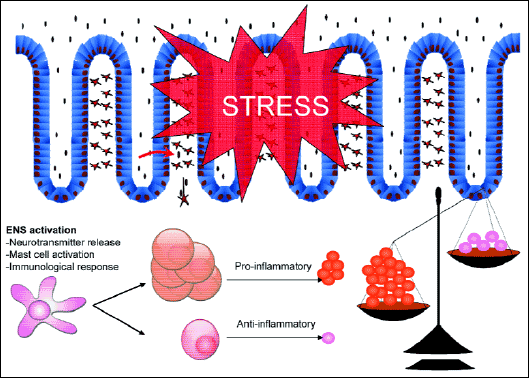 |
Fig. 5. Stress as exacerbating factor in the pathogenesis of inflammatory bowel disease. There is a strong evidence that the exposure to stress may lead to the exacerbation of inflammatory bowel disease. The exact mechanism responsible for this phenomenon is still not fully understood, but the stress leads to the shift toward increased expression of proinflammatory cytokines in colonic mucosa. |
There is also evidence that stress may have a profound effect on bacterial flora leading to increased adhesion and translocation of bacteria due to increased barrier permeability. This may be an important factor leading to the activation of the immune system resulting in the exacerbation or induction of acute colitis (39). Importantly, this effect could be alleviated by probiotics or antibiotics.
One of the most important diseases of the GI tract that is linked to stress exposure to gut is IBS, which represents a common, but heterogeneous gastrointestinal disorder with a worldwide prevalence of between 10-20%. Females are more commonly involved than males (F:M; 2:1 ratio). IBS is a functional disease and its diagnosis is mainly based on the exclusion of organic disease. IBS is characterized by periods of flare-ups and periods of remission. The most common symptoms are diarrhoea, constipation, abdominal pain and bloating (40-41).
Among important risk factors is genetic susceptibility and chronic stress (life events) while the key trigger factors include psychosocial factors and exposure of the gut to infections or overuse of antibiotics leading to negative alterations in gut flora. There is a strong evidence of the putative role of gut microbiota in the disturbance of brain-gut axis in IBS. Later during the course of this disease due to anxiety and the upregulation of immune system an irreversible state of self-perpetuation takes place (42).
The definition of IBS is based on the so called Rome III criteria which characterize IBS (43) if: recurrent abdominal pain or discomfort for
-improvement of symptoms with defecation;
-onset associated with a change in stool frequency;
-onset associated with a change in stool form (appearance);
The pathogenesis of IBS is multifactorial and in IBS patients a profound dysregulation of the brain gut axis takes place (Fig. 6). Among the observed gut disorders, the most important is a visceral hypersensitivity. In addition, release of important neuropeptides, up-regulation of immune system (low level inflammation), changes in motility, behaviour, endocrine functions and microbiota occur (44). IBS is classified into different subtypes based on the predominant symptoms: IBS-D, IBS-C, IBS-M (44).
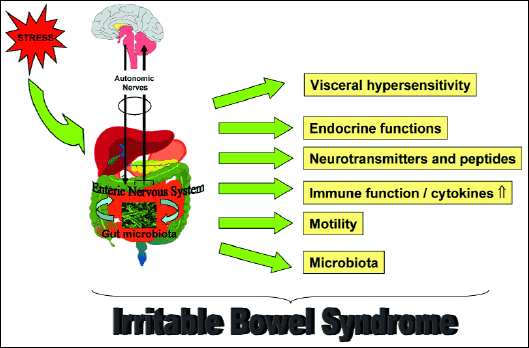 |
Fig. 6. Disruption of brain-gut-microbiota axis as the potential pathophysiological cause of irritable bowel syndrome. Stress is associated with the development of irritable bowel disease. The activation of brain-gut axis leads to the changes in visceral sensitivity, changes in the release of gastrointestinal hormones and neurotransmitters, increased expression and release of proinflammatory cytokines, changes in the gastrointestinal motility and gut microbiota. |
Diagnostic approach to the potential IBS patient includes obtaining a full medical history, physical examination, laboratory testing and operative diagnostic procedures. With concerns to taking the medical history, the physician should focus on the predominant bowel disorder for example diarrhoea, constipation, pain, bloating. From a diagnostic point of view, it is very important to associate the symptoms to an exposure to stress. Different stressors (physiological or physical like infection) may lead to flare-up or exacerbation of complaints. The physician should ask for the changes in the weight. It should be noted that most IBS patients have a stable weight. Since gastroenteritis may play an important role as a trigger in the development of IBS, the physician should ask whether the patient had the gastroenteritis in the past history. A careful history and physical examination may reveal clues that suggest a coexistence or alternative diagnosis, such as small intestinal bacterial overgrowth or celiac disease (CD).
Finally, the alarm symptoms ("red flags") such as unintentional weight loss, nocturnal diarrhoea, anaemia, bloody stools, onset of symptoms at 50 years or older should immediately prompt the physician to consider an alternative diagnosis, such as colon cancer. Sometimes, IBS patients may complain about extraintestinal symptoms. After taking a complete medical history of the patient, a through physical examination of the patients should be performed (45-47).
The differential diagnosis of IBS symptoms is broad and can lead to multiple, but often unnecessary, diagnostic tests. The laboratory testing in patients with suspected IBS should include: 1) routine blood tests (complete blood count, chemistries, CRP); 2) stool tests; 3) celiac disease serology (detection of transglutaminase-IgA), 4) thyroid function tests and 5) detection of calprotectin/lactoferrin in stool.
Finally, in addition to laboratory evaluation, new and modern diagnostic methods should be performed which include: 1) sonography; 2) gynaecological investigation in women, 3) colonoscopy (should be performed, especially in patients over the age of 50 to exclude organic disease of the colon); 3) H2 breath tests for exclusion of carbohydrate maldigestion (lactose, fructose) or small intestinal bacterial overgrowth (SIBO) (48) (Fig. 7).
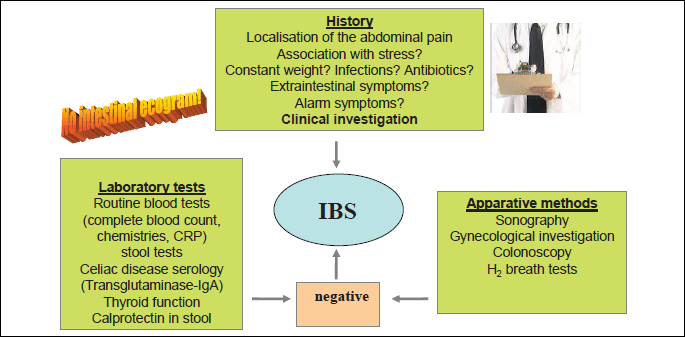 |
| Fig. 7. Irritable bowel syndrome (IBS) is generally diagnosed on the basis of a complete medical history that includes a careful description of symptoms and a physical examination. No specific tests for diagnosis for IBS exist. To exclude other important diseases some laboratory tests including routine blood tests, stool sample testing, celiac disease serology and thyroid function tests are recommended. Concerning the apparative methods, the most important role play the abdominal sonography and colonoscopy (especially in patients with diarrhea dominant IBS). In women the gynaecological investigation is recommended. Finally, it is important to exclude the lactose-, fructose- or sorbitol malabsorption or small bowel bacterial overgrowth using H2 breath tests. |
The therapy of IBS includes: 1) general measures: 2) pharmacotherapy and 3) psychological and behavioural therapy. One of the most important aspects in the management of IBS is the development of positive physician-patient relationship. The impact of IBS symptoms on patients is often associated with feelings of shame, fearfulness or embarrassment, which patients perceive to be poorly understood by physicians or family. The positive physician-patient relationship can significantly increase the efficiency of the therapy. In other words, providing more information to the patient and reassuring that IBS represents a functional disease of the gut, and may play an important role in the therapy of IBS.
A lot of patients believe that diet plays an important role in the exacerbation of symptoms. Dietary assessment plays important role, and the symptoms may improve after avoiding of following: milk products, some carbohydrates (sorbitol, fructose), caffeine and alcohol. Eventually screening for food intolerance or allergy should be performed. Response to exclusion diets varied from 15-70% in dependence of the study. Patients with IBS have increased prevalence of lactose, fructose or sorbitol intolerance (49). Generally, for IBS patients a graduated treatment approach is recommended. For mild symptoms, education, reassurance and dietary adjustment may suffice. For moderate symptoms, identification and modification of exacerbating factors, psychotherapeutic and behavioural techniques aimed at the predominant symptoms are recommended. In the severe forms, multidisciplinary approach and pharmacotherapy are needed. Sometimes, referral to specialized pain treatment centres are requested (40).
Pharmacotherapy should be tailored to the leading symptoms. However, a symptom-based therapy does not modify the natural history of the disorder. The recent advances in the elucidation of the pathogenesis of IBS have resulted in the development of novel therapies. The most important therapies at the moment include serotonergic drugs. These groups of drugs were reported to modulate the activity of serotonin in the gut. Serotonin stored in enterochromaffin cells (ECC) plays a crucial role in the motility, visceral sensitivity and secretion. Further drugs effective in IBS treatment include antimuscarinic agents, µ-opioid antagonists, CRF antagonists, chloride channel opener and even melatonin (50). This last group of drugs show a positive effect on IBS via antioxidative, anti-inflammatory and anti-motility effects. Melatonin strongly prevents the exacerbation of colitis caused by stress as shown in appropriate animal models (51).
Pharmacotherapy should be based on the leading symptoms. Treatment options for IBS with the predominant symptom being diarrhoea (IBD-D) include anti-diarrhoeals such as loperamide (1-8 mg for times daily in divided doses). It is important to titrate dose for desired effect to side effects such as constipation. Although loperamide is useful for the treatment of diarrhoea, no global symptom relief can be expected under this medication (51). Interestingly, the 5-HT3-antagonists such as alosetron 0.5-1 mg twice daily have been shown to be effective in the treatment of diarrhoea in IBS patients. However, the serious complications, especially ischemic colitis limited its use and led to the withdrawal of this medication from the market in Germany. However, in the USA alosetron is available for the treatment of severe IBS-D in women under an appropriate risk management program (52, 53). Recently, Pimentel et al. (54) demonstrated that luminal acting antibiotic rifaximin showed a global efficacy and significant improvement of bloating in IBS-D patients. Moreover, tricyclic antidepressant (amitryptiline 10-150 mg at night; doxepin 10-130 mg at night, trimipramine 10-150 mg at night or desipramine 10-150 mg at night etc.) can be recommended in some IBS-D patients. It is important to initiate the therapy at a lower dose than the typical dose given for mood disorders (48). Finally, recent studies indicate that some probiotics may be useful in the therapy of diarrhoea in IBS patients. However, the exact dose of probiotics requires evaluation with more number of studies (48).
Treatment options for IBS patients with constipation (IBS-C) include the bulking agents (psyllium 2.5-30 g daily in divided doses) and laxatives (polyethylene glycol, PEG) (55, 56). Prucalopride, which is a selective 5-HT4 agonist given 1-2 mg once daily, relieved symptoms of constipation in patients with IBS-C (57). The activators of mucosal epithelial chloride channels play an important role in fluid transportation within the colon and may be successfully implemented in the therapy of IBS patients with constipation. Lubiprostone, with an approved IBS-C patients dose of 8 µg twice daily is a prostaglandin E1 derivative which activates the mucosal epithelial chloride channels and promotes chloride-rich fluid secretion into the lumen of GI tract. This leads to softening of the stool and acceleration of colonic transit (58). Finally, there is also evidence that some probiotic strains may be useful in IBS-C patients (59).
In IBS patients with bloating and pain the recommended first line of therapy is a antispasmodics such as hyoscamine sulphate (0.125 up to four times daily) or dicyclomine (10-10 mg twice daily up to four times daily). Antispasmodics act predominantly as antagonists at cholinergic receptors and thereby reduce contraction of the GI tract. In addition to reduction of colonic contraction by antispasmodics, tricyclic antidepressants (TCA) and selective serotonin reuptake inhibitors SSRI such as fluoxetine (10-40 mg daily), citalopram (20 mg daily), sertraline (25-100 mg daily) and escitalopram (10 mg daily) are effective in the treatment of chronic pain in IBS patients. At the molecular level, TCA inhibit the re-uptake of both serotonin and norepinephrine, increasing the bioavailability of these neurotransmitters in the synaptic cleft. In contrast, SSRI prevent the re-uptake of serotonin alone (60). In recent years alpha 2 delta (a2d) ligands, gabapentin and pregabalin have been shown to reduce visceral pain perception in IBS patients (61). Finally, pre- and probiotics may positively influence the visceral hypersensitivity and reduce bloating in IBS patients. However, the clinical benefits seem to be strain-dependent (62).
Recently, an increasing number of studies indicates the positive effect of probiotics IBS on symptoms. The postulated mechanisms of action of probiotics on stressed gastrointestinal mucosa include: 1) improvement of the barrier function of the epithelium (permeability); 2) suppression of the growth and binding of pathogenic bacteria; 3) positive effect on visceral hypersensitivity and 4) immunomodulatory effect (inhibition of subclinical inflammation in IBS). Despite the high number of studies using probiotics in IBS patients, a lot of new questions have been left unanswered such as the optimal dose, its role in combination therapy, strain specific activity, stability within GI tract, possible development of antibiotic resistance and the duration of therapy. Probiotics may vary in species, strains, preparation and doses, what makes the interpretation of the efficacy difficult. To answer all these questions, large placebo-controlled trials are needed in the future.
In some clinical settings, a combination therapy approach could be recommended. However, a physician combining different types of drugs should be aware of possible side effects and interactions. Combination therapy should be aimed at the predominant symptoms of the patient (63).
Complementary or alternative approaches includes cognitive behavioural therapy (CBT), dynamic psychotherapy, stress management, hypnotherapy, relaxation therapy and even acupuncture represent further important treatment approaches to reduce the symptoms of IBS (64, 65). These forms of therapy should not only be used in refractory forms of disease, but also used as useful adjunctive therapy.
We conclude that 1) exposure to stress (especially chronic stress) is a major risk factor in the pathogenesis of different diseases of gastrointestinal tract including gastroesophageal reflux disease (GERD), peptic ulcer, functional dyspepsia, inflammatory bowel disease (IBD), irritable bowel disease (IBS), and other functional disorders of GI tract; 2) the dysregulation of brain-gut-axis plays a central role in the pathogenesis of stress-induced diseases; 3) Stress increases intestinal permeability, visceral sensitivity, alteration in GI-motility and leads to profound mast cell activation resulting in release of many proinflammatory mediators; 4) diagnostic approach to stress-induced gastrointestinal disorders includes: laboratory tests, imaging techniques (abdominal sonography), endoscopy (gastroscopy, colonoscopy, small intestine endoscopy) as well as H2 breath test for exclusion of small intestinal bacterial overgrowth (SIBO) and maldigestion of carbohydrates; 5) Therapy is based mainly on the leading symptoms and includes general measurements, pharmacotherapy as well as psychological and behavioural therapies.
Conflict of interests: None declared.
- Selye H. Syndrome produced by diverse nocuous agents. Nature 1936; 138: 32.
- Bhatia V, Tandon RK. Stress and the gastrointestinal tract. J Gastroenterol Hepatol 2005; 20: 332-339.
- Soderholm JD, Perdue MH. Stress and gastrointestinal tract. II. Stress and intestinal barrier function. Am J Physiol Gastrointest Liver Physiol 2001; 280: G7-G13.
- Nakade Y, Fukuda H, Iwa M, et al. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am J Physiol Gastrointestinal Liver Physiol 2007; 292: G1037-G1044.
- Konturek SJ, Brzozowski T, Konturek PC, Zwirska-Korczala K, Reiter RJ. Day/night differences in stress-induced gastric lesions in rats with an intact pineal gland or after pinealectomy. J Pineal Res 2008; 44: 408-415.
- Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res 2011; 343: 23-32.
- Beaumont W. Experiments and Observations on the Gastric Juice and the Physiology of Digestion. Edinburgh, Maclachlan and Stewart, 1838.
- Laranjeira C, Pachnis V. Enteric nervous system development: recent progress and future challenges. Auton Neurosci 2009; 151: 61-69.
- Konturek SJ, Konturek JW. Pawlik T, Brzozowski T. Brain-gut axis and its role in the control of food intake. J Physiol Pharmacol 2004; 55: 137-154.
- Mayer EA. Tilisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med 2011; 62: 381-396.
- Bonaz B, Sabate JM, Brain-gut axis dysfunction. Gastroenterol Clin Biol 2009; 33(Suppl. 1): S48-S58.
- Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest 2007; 117: 33-40.
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009; 6: 306-314.
- Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res 2011; 343: 23-32.
- Konturek PC, Sliwowski Z, Koziel J, et al. Probiotic bacteria Escherichia coli strain Nissle 1917 attenuates acute gastric lesions induced by stress. J Physiol Pharmacol 2009; 60(Suppl 6): 41-48.
- Farhadi A, Fields JZ, Keshavarzian A. Mucosal mast cells are pivotal elements in inflammatory bowel disease that connect the dots: stress, intestinal hyperpermeability and inflammation. World J Gastroenterol 2007; 13: 3027-3030.
- Wallon C, Yang PC, Keita AV, et al. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut 2008; 57: 50-58.
- Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut 2010; 59: 489-495.
- O'Malley D, Quigley EM, Dinan TG, Cryan JF. Do interactions between stress and immune responses lead to symptom exacerbations in irritable bowel syndrome? Brain Behav Immun 2011; 25: 1333-1341.
- Stasi C, Orlandelli E. Role of the brain-gut axis in the pathophysiology of Crohn's disease. Dig Dis 2008; 26: 156-166.
- Yang PC, Jury J, Söderholm JD, Sherman PM, McKay DM, Perdue MH. Chronic psychological stress in rats induces intestinal sensitization to luminal antigens. Am J Pathol 2006; 168: 104-114.
- Mittal RK, Stewart WR, Ramahi M, Chen J, Tisdelle D. The effects of psychological stress on the esophagogastric junction pressure and swallow-induced relaxation. Gastroenterology 1994; 106: 1477-1484.
- Mizyed I, Fass SS, Fass R. Review article: gastro-oesophageal reflux disease and psychological comorbidity. Aliment Pharmacol Ther 2009; 29: 351-358.
- Li J, Brackbill RM, Stellman SD, et al. Gastroesophageal reflux symptoms and comorbid asthma and posttraumatic stress disorder following the 9/11 terrorist attacks on World Trade Center in New York City. Am J Gastroenterol 2011; 106: 1933-1941.
- Perlman SE, Friedman S, Galea S, et al. Short-term and medium-term health effects of 9/11. Lancet 2011; 378: 925-934.
- Yeomans ND. The ulcer sleuths: the search for the cause of peptic ulcers. J Gastroenterol Hepatol 2011; 26(Suppl 1): 35-41.
- Konturek PC. Physiological, immunohistochemical and molecular aspects of gastric adaptation to stress, aspirin and to H. pylori-derived gastrotoxins. J Physiol Pharmacol 1997; 48: 3-42.
- Ali T, Harty RF. Stress-induced ulcer bleeding in critically ill patients. Gastroenterol Clin North Am 2009; 38: 245-265.
- Konturek PC, Brzozowski T, Burnat G, et al. Role of brain-gut axis in healing of gastric ulcers. J Physiol Pharmacol 2004; 55: 179-192.
- Brzozowski T, Konturek PC, Pajdo R, et al. Importance of brain-gut axis in the gastroprotection induced by gastric and remote preconditioning. J Physiol Pharmacol 2004; 55: 165-177.
- Loyd RA. McClellan DA. Update on the evaluation and management of functional dyspepsia. Am Fam Physician 2011; 83: 547-552.
- Kachintorn U. Epidemiology, approach and management of functional dyspepsia in Thailand. J Gastroenterol Hepatol 2011; 3: 32-34
- Tack J, Talley NJ. Gastroduodenal disorders. Am J Gastroenterol 2011; 105: 757-763.
- Camillieri M, Tack JF. Current medical treatments of dyspepsia and irritable bowel syndrome. Gastroenterol Clin North Am 2010; 39: 481-493.
- Singh S, Graff LA, Bernstein CN. Do NSAIDs, antibiotics, infections, or stress trigger flares in IBD? Am J Gastroenterol 2009; 104: 1298-1313.
- Li J, Norgard B, Precht DH, Olsen J. Psychological stress and inflammatory bowel disease: a follow-up study in parents who lost a child in Denmark. Am J Gastroenterol 2004; 99: 1129-1133.
- Israeli E, Hershcovici T, Berenshtein E, et al. The effect of restraint stress on the normal colon and on intestinal inflammation in a model of experimental colitis. Dig Dis Sci 2008; 53: 88-94.
- Camara RJ, Ziegler R, Begre S, Schoepfer AM, von Kanel R. Swiss inflammatory bowel disease cohort study (SIBDCS) group. The role of psychological stress in inflammatory bowel disease: quality assessment of methods of 18 prospective studies and suggestions for future research. Digestion 2009; 80: 129-139.
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 2011; 25: 397-407.
- Khan S, Chang L. Diagnosis and management of IBS. Nat Rev Gastroenterol Hepatol 2010; 7: 565-581.
- Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci 2011; 12: 453-466.
- Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Ann Rev Med 2011; 62: 381-396.
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006; 130: 1480-1491.
- Clarke G, Quigley EM, Cryan JF, Dinan TG. Irritable bowel syndrome: towards biomarker identification. Trends Mol Med 2009; 15: 478-489.
- Hammerle CW, Crowe SE. When to reconsider the diagnosis of irritable bowel syndrome. Gastroenterol Clin North Am 2011; 40: 291-307.
- Rubin G, De Wit N, Meineche-Schmidt V, Seifert B, Hall N, Hungin P. The diagnosis of IBS in primary care: consensus development using nominal group technique. Fam Pract 2006; 23: 687-692.
- Zimmerman J. Extraintestinal symptoms in irritable bowel syndrome and inflammatory bowel diseases: nature, severity, and relationship to gastrointestinal symptoms. Dig Dis Sci 2003; 48: 743-749.
- Keller J, Wedel T, Seidl H, et al. S3 guideline of the German Society for Digestive and Metabolic Diseases (DGVS) and the German Society for Neurogastroenterology and Motility (DGNM) to the definition, pathophysiology, diagnosis and treatment of intestinal motility. Z Gastroenterol 2011; 49: 374-390.
- Grundmann O, Yoon SL. Irritable bowel syndrome: epidemiology, diagnosis and treatment: an update for health-care practitioners. J Gastroenterol Hepatol 2010; 25: 691-699.
- Chang JY, Talley NJ. Current and emerging therapies in irritable bowel syndrome: from pathophysiology to treatment. Trends Pharmacol Sci 2010; 31: 326-334.
- Konturek PC, Brzozowski T, Konturek SJ. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol 2011; 62: 139-150.
- Bleser S. Alosetron for severe diarrhea-predominant irritable bowel syndrome: improving patient outcomes. Curr Med Res Opin 2011; 27: 503-512.
- Lewis JH. Alosetron for severe diarrhoea-predominant irritable bowel syndrome: safety and efficacy in perspective. Expert Rev Gastroenterol Hepatol 2010; 4: 13-29.
- Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011; 364: 22-32.
- Chey WD, Maneerattaporn M, Saad R. Pharmacologic and complementary and alternative medicine therapies for irritable bowel syndrome. Gut Liver 2011; 5: 253-266.
- Awad RA, Camacho S. A randomized, double-blind, placebo-controlled trial of polyethylene glycol effects on fasting and postprandial rectal sensitivity and symptoms in hypersensitive constipation-predominant irritable bowel syndrome. Colorectal Dis 2010; 12: 1131-1138.
- Manabe N, Rao AS, Wong BS, Camilleri M. Emerging pharmacologic therapies for irritable bowel syndrome. Curr Gastroenterol Rep 2010; 12: 408-416.
- Camilleri M. New receptor targets for medical therapy in irritable bowel syndrome. Aliment Pharmacol Ther 2010; 31: 35-46.
- Pohl D, Tutuian R, Fried M. Pharmacologic treatment of constipation: what is new? Curr Opin Pharmacol 2008; 8: 724-728.
- Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol 2009; 15: 1548-1553.
- Gale JD, Houghton LA. Alpha 2 delta (a(2)d) ligands, gabapentin and pregabalin: what is the evidence for potential use of these ligands in irritable bowel syndrome. Front Pharmacol 2011; 2: 28.
- Whelan K. Probiotics and prebiotics in the management of irritable bowel syndrome: a review of recent clinical trials and systematic reviews. Curr Opin Clin Nutr Metab Care 2011; 14: 581-587.
- Sainsbury A, Ford AC. Treatment of irritable bowel syndrome: beyond fiber and antispasmodic agents. Therap Adv Gastroenterol 2011; 4: 115-127.
- Yoon SL, Grundmann O, Koepp L, Farrell L. Management of irritable bowel syndrome (IBS) in adults: conventional and complementary/alternative approaches. Altern Med Rev 2011; 16: 134-151.
- Magge S, Lembo A. Complementary and alternative medicine for the irritable bowel syndrome. Gastroenterol Clin North Am 2011; 40: 245-253.