HUMAN SKELETAL MUSCLE-DERIVED STEM/PROGENITOR CELLS MODIFIED WITH CONNEXIN-43 PREVENT ARRHYTHMIA IN RAT POST-INFARCTION HEARTS AND INFLUENCE GENE EXPRESSION IN THE MYOCARDIUM
4Department of Clinical Pathology, Poznan University of Medical Sciences, Poznan, Poland
The genetic modification of human skeletal muscle-derived stem/progenitor cells with connexin-43 prevented the pro-arrhythmic effects of myogenic implanted stem cells on the host myocardium and positively influenced myocardial gene expression profiles in respect to myocardium conductivity.
INTRODUCTION
The heart is the principal organ in the human body responsible for blood circulation. Cardiac disorders are among the most common causes of human morbidity and mortality worldwide (1). According to the latest reports from the World Health Organization (WHO), approximately 17.5 million people died from cardiac diseases alone in 2012, and currently, heart failure is emerging as the number one problem among cardiovascular disorders. Therefore, novel treatments are urgently needed.
Cellular therapies including skeletal muscle myogenic precursors are an alternative method for heart failure treatment. Due to the very promising results obtained with skeletal myoblasts in pre-clinical observations, these cells were one of the first used in clinical trials (2, 3). Moreover, we belonged to one of the first groups applying autologous skeletal myoblasts for treatment of postinfarction heart (4, 5). Skeletal myoblasts appear to have many potential advantages compared with other types of stem cells. Under in vitro culture conditions, myoblasts demonstrated the capacity for stable and rapid proliferation, and under in vivo conditions, these cells appeared to be more resistant to ischaemia than other stem cells; additionally, these cells can be easily obtained from adult individuals (6).
However, when preparing myoblasts for cell intervention, propagated in vitro cells had in situ adverse heterogenic action potentials along the cell membrane, which might be due to differences in the duration of action potentials that arise between the implanted ‘native’ myoblasts and cardiomyocytes from the recipient heart (7). This phenomenon occurs due to the lack of functional ‘gap junctions’ between these two types of cells (8, 9). The ‘gap junctions’ are formed by connexin-43 protein hemichannels, and they allow ions and small molecules to pass from one cell to another via intracellular communication. As you can see in Fig. 1, human myoblasts are characterized by the presence of CX43 (connexin-43) protein. However, when implanted into a post-infarcted heart, they differentiate into mature cells - myotubes, which do not present the connexin-43 protein. Consequently, heart arrhythmia may readily occur due to insufficient electrophysiological synchronization with the recipient organ cardiomyocytes. Therefore, the implantation of skeletal muscle stem/progenitor cells can lead to cardiac ventricular arrhythmias.
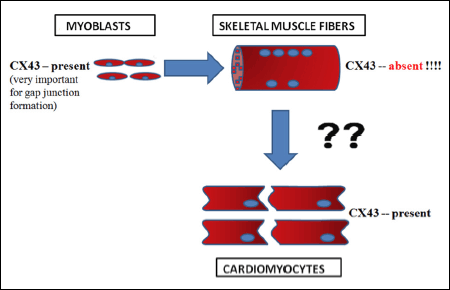 |
Fig. 1. The possible cause of arrhythmia after implanting human ‘native’ myoblasts into post-infarcted myocardium. The connexin-43 protein (CX43) was present in the myoblasts and cardiomyocytes, but not in the mature myotubes. Thus, after implanting the myoblasts in the post-infarcted heart, arrhythmia may occur due to insufficient electrophysiological synchronization with the recipient organ cardiomyocytes. |
We hypothesized that genetic modification with a gene encoding ‘gap junction’ formation may eliminate this phenomenon. Therefore, SkMDS/PCs were modified with the gene encoding connexin-43, the main protein responsible for ‘gap junction’ development (10, 11).
We assumed that SkMDS/PCsCX43 would improve communication between implanted cells and host cardiomyocytes, permitting better ion flow between these cells, which would consequently provide synchronous action potentials, thus reducing the number of arrhythmogenic events in the post-infarction heart.
In the current study, we employed a rat myocardial infarction (MI) model to investigate whether SkMDS/PCsCX43 can exert a protective effect against myocardial dysfunction, such as the post-infarction arrhythmias that occur after the transplantation of SkMDS/PCsWT into the post-infarction heart.
We evaluated the effects of SkMDS/PCsCX43 on pro-arrhythmic episodes in a rat model of post-infarcted heart. Next, we performed gene and protein expression analyses on selected gene clusters associated with arrhythmia (Kcnq1, Ncx1, Cacna1c, and Serca2a) and left ventricle remodelling (Serca2a, Gata4, and Tgfb1). To the best of our knowledge, this study is the first to compare the expression profiles of gene clusters associated with arrhythmias (at the transcriptional level) and their respective protein products in the myocardium in situ.
MATERIALS AND METHODS
The Local Bioethics Committee at the Poznan University of Medical Sciences approved the protocol for human tissue collection that was subsequently used for human cell intervention in the animal model and written consents from the patients were obtained. The Local Ethical Committee for Animal Research at Poznan University of Life Sciences approved the protocol for the experiments performed in the rat post-infarction heart model. All animal experiments were performed in accordance with relevant guidelines and regulations.
Human skeletal muscle-derived stem/progenitor cells isolation, characteristics and in vitro culture
Human SkMDS/PCs were obtained from muscle tissue fragments derived from posterior muscle group of quadriceps after surgical reconstruction of the anterior cruciate ligaments. For this purpose, an approval from the Local Bioethics Committee (Poznan University of Medical Sciences) was granted. Isolation of SkMDS/PCs was performed according to a previously described pre-plate technique with minor modifications (12). Cells were then in vitro cultured in standard Dulbecco’s modified Eagle’s medium with 4.5 g/l of glucose supplemented with 20% foetal bovine serum (FBS), 1% antibiotics, 1% ultraglutamine (Lonza Group, Basel, Switzerland) and other routine supplements. Additionally, bFGF (Sigma-Aldrich, St. Louis, USA) was added to the medium as previously described (12). SkMDS/PCs were incubated under standard in vitro culture conditions (5% CO2, 37ºC, and 95% humidity), and the medium was changed every other day. After the cells reached ~80% confluence, they were passaged and detached from the flask using a 0.25% trypsin/EDTA solution (Gibco, Life Technologies, Warsaw, Poland).
During cell culture the cells were tested with fluorochrome-conjugated antibody for the identification of the cell population expressing CD56 marker using flow cytometry according to manufacturer’s manual (Beckman-Coulter, Brea, USA). Briefly, to the pellet of 250,000 cells reuspended in 100 µl PBS with 2% FBS, 10 µl of anti-CD56 antibody was added and mixed thoroughly. After a 20-minute incubation in the dark, 1 ml PBS + 2% FBS was added and centrifuged for 5 minutes. Then, after decanting the supernatant, 1.5 ml PBS + 2% FBS to the cell pellet was again added and centrifuged for another 5 minutes. Finally, the cells were resuspended in 500 µl PBS with 2% FBS and analyzed in a flow cytometry.
To confirm the myogenic characteristics of the studied cell populations the determination of desmin, intermediate filaments was performed. In brief, the fixed slides with cells were incubated for 15 minutes in a solution of 0.1% Triton X-100 in PBS to permeabilize the cell membranes. After washing in PBS, non-specific epitopes were blocked incubating the slides with 1% serum in PBS (25 μl /well) in a humidified chamber for 10 minutes at room temperature.
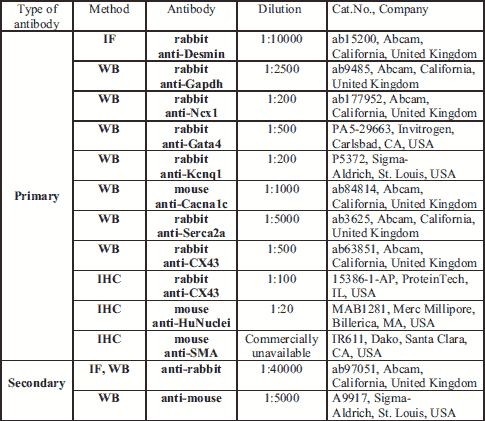
Next, incubation with primary antibody anti-desmin (Abcam, Cambridge, United Kingdom) in 1% BSA in PBST in a humidified chamber overnight at 4ºC was performed. After incubation, the cells were subjected to a secondary antibody Goat Anti-Rabbit IgG (Abcam, Cambridge, United Kingdom) diluted in 1% BSA for 1 hour at room temperature in the dark. The antibodies are listed in Table 1.
Plasmid
A pCiNeo plasmid (Promega, Fitchburg, WI, USA) with the coding sequence for the GJA1 gene was constructed at the Institute of Human Genetics, Polish Academy of Sciences in Poznan, Poland, as previously described (11).
in vitro transfection and efficiency
Cells were transiently transfected with the connexin-43 gene using electroporation. Conditions for this procedure were optimized in our previous experiments (13). In further experiments, the cells were incubated under standard in vitro culture conditions as previously described. After 48 hours, the cells were treated with 4% (v/v) paraformaldehyde for 15 min incubated with 0.1% (v/v) Triton X-100 for 15 min, and blocked with 10% (w/v) goat serum for 1 hour, with PBS washes after each step. Subsequently, the cells were incubated with a rabbit anti-connexin-43 antibody (Abcam, Cambridge, UK) at 4ºC o/n, labelled with an Alexa Fluor 594 donkey anti-goat secondary antibody (Abcam, Cambridge, UK) for 1 hour, and examined using fluorescence microscopy (model DMi8, Leica, Morrisville, USA).
Western blot analysis
Connexin-43 expression levels were evaluated with respect to the reference protein β-actin (ACTB) by Western immunoblotting. Briefly, proteins were isolated from cell pellets using an AllPrep DNA/RNA/Protein Mini Kit (Qiagen, Venlo, Netherlands), and equal protein aliquots (100 µg) were separated by IPA gel electrophoresis. The separated proteins were blotted onto PVDF Transfer Membranes (EMD Millipore Corporation, Billerica, MA USA). The blots were then probed with antibodies against connexin-43 (Abcam, Cambridge, UK) and developed with anti-rabbit antibodies conjugated to horseradish peroxidase (Abcam, Cambridge, UK). Semi-quantitative analysis was performed using a Molecular Imager ChemiDoc XRS + System (Bio-Rad, Hercules, CA, USA). The measurements were normalized to ACTB.
For the heart tissue samples, 50-μg protein aliquots were separated on 4 – 20% Tris-glycine gels (BioRad, Hercules, CA, USA). After electrophoresis, the proteins were blotted onto PVDF Transfer Membranes (EMD Millipore Corporation, Billerica, MA, USA). PVDF blots were blocked for 1 hour with 5% non-fat dry milk/Tris Buffered Saline + Tween (5% NFDM/TTBS) at room temperature, followed by an overnight incubation at 4ºC with one of the following primary antibodies: (1) anti-Ncx1 (Abcam, Cambridge, UK), (2) anti-Cacna1c (Abcam, Cambridge, UK), (3) anti-Kcnq1 (Sigma-Aldrich, St. Louis, MO, USA), (4) anti-Serca2a (Abcam, Cambridge, UK), (5) anti-Gata4 (Invitrogen, Carlsbad, CA, USA), or (6) anti-Gapdh (Abcam, Cambridge, UK) (Table 1). The blots were washed three times with TTBS (3 × 10 min) and incubated for 1 hour at room temperature with one of the following secondary antibodies: (1) goat anti-rabbit IgG (Abcam, Cambridge, UK) or (2) anti-mouse IgG for Cacna1c. The blots were then washed three more times, twice with TTBS and once with 1 × TBS, before they were developed using a BioRad Clarity ECL Kit. Imaging was performed using the BioRad Gel Documentation System and its accompanying software. Measurements were normalized to Gapdh expression.
Immunohistochemical staining
For the immunohistochemical investigations, we used histological heart sections collected from all the animal groups included in the study with the following intracardial interventions: 1) MI + SkMDS/PCsCX43, 2) MI + SkMDS/PCsWT and 3) MI + 0.9%NaCl. The experiments were conducted using mouse anti-human nucleus (HuNuclei), rabbit anti-connexin-43, and mouse anti-alfa-smooth muscle actin (SMA) antibodies (Table 1). The Dako EnVisionTM FLEX detection system was used to detect primary mouse and rabbit antibodies in the immunohistochemical preparations according to the manufacturer’s instructions. Universal Negative Control for IR-series of Rabbit Primary Antibodies (Cat No IR600) and Universal Negative Control for IR series of Mouse Primary Antibodies (Cat No IR750) from Dako/Perlan, via Real, CA, USA) served as negative antibody isotype control staining.
The samples were fixed in a 10% formalin solution for 48 hours with subsequent standard paraffin block embedding. Five-micrometre histological sections were cut using a microtome, and then standard haematoxylin and eosin staining was performed. Tissue sections (5 µm) from formalin-fixed paraffin-embedded specimens were mounted on IHC Microscopic Slides (DAKO, Santa Clara, CA, USA).
For smooth muscle actin staining, the sections were deparaffinized, rehydrated and heated at 97ºC for 20 min in EnVisionTM FLEX Target Retrieval Solution with high pH (DAKO, Santa Clara, CA, USA) for antigen retrieval. SMA immunostaining was performed using an AutostainerLink48 system (DAKO, Santa Clara, CA, USA) under the following conditions: 1) incubation with the EnVisionTM FLEX Peroxidase-Blocking Reagent for 5 min, 2) incubation with mouse anti-SMA primary antibody for 20 min at room temperature and the EnVisionTM FLEX/HRP secondary antibody for 20 min, and 3) incubation with the EnVisionTM FLEX Substrate Working Solution for an additional 10 minutes. Sections were counterstained with Mayer’s hematoxylin, dehydrated, cleared and mounted in DPX.
For connexin-43 and human nuclei membranes staining, the sections were deparaffinized, rehydrated and heated at 97ºC for 20 min in EnVisionTM FLEX Target Retrieval Solution with low pH (DAKO, Santa Clara, CA, USA). The sections were then incubated with the EnVisionTM FLEX Peroxidase-Blocking Reagent (DAKO, Santa Clara, CA, USA) for 5 minutes. Primary antibodies specific for connexin-43 (ProteinTech, #15386-1-AP, GJA1) and HuNuclei were added at their optimal dilutions (Table 1), and the samples were incubated at 4ºC overnight (connexin-43) or for 1 hour at room temperature (HuNuclei). The sections were then incubated with the EnVisionTM FLEX/HRP secondary antibody (DAKO, Santa Clara, CA, USA) for 20 min, and signal detection was performed using the EnVisionTM Substrate Working Solution (DAKO, Santa Clara, CA, USA). Sections were counterstained with Mayer’s hematoxylin, dehydrated, cleared and mounted in DPX.
Animals
The experiments were performed using female Wistar rats weighing 200 – 250 g that were purchased from the Center for Experimental Medicine, Bialystok Medical Sciences University, as previously described (14). The rats were maintained in animal facilities at the Institute of Human Genetics, Polish Academy of Sciences, Poznan, Poland.
Experimental design
Fig. 2 illustrates the animal experimental protocol. On day 1, a myocardial infarction was produced. Following surgery, the animals were divided into the following three subgroups: (i) group with ‘native’ SkMDS/PCs intervention (MI + SkMDS/PCsWT) (n = 12), (ii) control group with ‘sham’ delivery of 0.9% saline solution (MI + 0.9%NaCl) (n = 12), and (iii) group with GJA1 gene-transfected SkMDS/PCs intervention (MI + SkMDS/PCsCX43), (n = 12). On day 14, intramyocardial interventions and echocardiographic (Echo) examinations were performed. From the first day of cell intervention, the animals were subjected to immunosuppression with cyclosporine (Sandimmun, Novartis, Basel, Switzerland) at a final concentration of 10 mg/kg body mass. To evaluate the anti-arrhythmic effects of the transplanted cells, cardiac arrhythmia was induced on day 42.
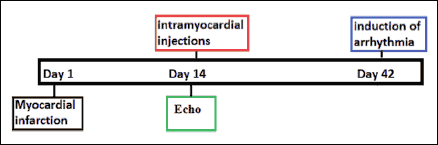 |
Fig. 2. Experimental protocol. Intramyocardial interventions were performed at 2 weeks after the induction of heart infarction due to left coronary artery ligation. Echocardiography (Echo) was performed 2 weeks after myocardial infarction (just before SkMDS/PCs delivery or the ‘sham’ intervention) and on the last day of the experiment. The induction of arrhythmia was performed 28 days after cell administration. |
Induction of myocardial infarction and Echo
Myocardial infarction was induced in 50 young female Wistar rats by ligating the left anterior descending coronary artery under isoflurane anaesthesia with 100% oxygen ventilation. Subsequently, the chest was closed and sutured, and the rats were allowed to recover.
Echocardiography was performed using a MyLab25 system (Esaote, Genova, Italy) with a 13 MHz linear array transducer at specific time points (see experimental protocol timeline; Fig. 2) to determine the extent of myocardial infarction. To quantitate the regional LV wall motion abnormalities, we applied the wall motion index (WMI). Rats with a WMI > 15 were considered to have had a large myocardial infarction. We found similar infarct sizes (no statistically significant differences) in all the animals subjected to infarction induction according to echocardiography performed.
Induction of cardiac arrhythmia
To induce ventricular tachycardia in rat hearts in situ, the animals were anesthetized with ketamine and xylazine and attached to a respirator. Then, the chest was opened, and the heart exposed. The sidewall of the left ventricle was then stimulated with a bipolar electrode connected to an external pacemaker. We 1) stimulated the chamber with a single frequency of 1 Hz for 2 min and then 2) with double the frequency of 1 Hz for a further 2 min; subsequently, stimuli with a single frequency of 3) 8 Hz for 4 × 10 seconds and (4) 15 Hz 4 × 10 seconds were applied. Ventricular tachycardia, ventricular fibrillation (VF), the total duration of ventricular arrhythmias and sustained settled tachycardia/fibrillation leading to animal death were recorded for each animal.
Heart tissue collection
The rats were terminated on the 42nd day of the experiment by cervical dislocation. Their isolated hearts were washed three times with a 0.9% NaCl solution, and the left ventricles were isolated and stored in RNALater (Ambion, Foster City, CA, USA) solution at –80ºC or in liquid nitrogen until immunohistochemical staining.
Sample preparation and gene expression analysis
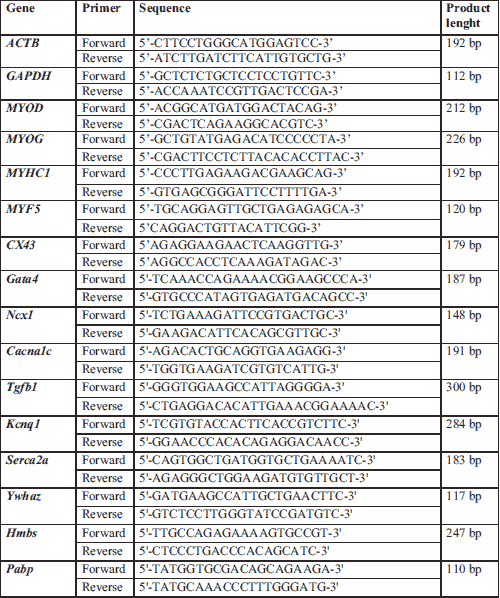
Real-time polymerase chain reaction was performed to assess the expression of connexin-43 and selected human myogenic genes (MYOD, MYOG, MYF5, and MYHC1) after transfecting human SkMDS/PCs with GJA1. Rat myocardium genes involved in arrhythmia (Kcnq1, Ncx1, Cacna1c) and heart remodelling (Serca2a, Gata4, and Tgfb1) were also investigated. The primers are listed in Table 2. Total RNA was isolated from in vitro cultured human SkMDS/PCs or rat left ventricular cells using an AllPrep Mini Kit (Qiagen, Venlo, Netherlands). RNA yield was determined using a Nanodrop 2000 (Thermo Scientific, Waltham, MA, USA), and its quality was assessed using standard 1.5% agarose gel electrophoresis. Afterwards, 5 µg of each RNA sample was used for reverse transcription to obtain cDNA template for qPCR. The following PCR thermocycling conditions were used for reverse transcription: 1) 20ºC for 10 min, 2) 37ºC for 1 hour and 3) 70ºC for 10 minutes. The real-time PCR quantitative reactions were performed using an iCycler detection system (Bio-Rad, Hercules, CA, USA). The results were normalized to the ACTB (for human samples) and Gapdh (for rat samples) reference genes.
The simultaneous extraction of RNA and protein from the same sample is critical for the accurate identification of correlations between genomic and proteomic expression (15). Our sample preparations were performed using either the total left ventricle from rat hearts or human SkMDS/PCs suspensions (with or without genetic modification). Each left heart ventricle was cut into small pieces, placed in 1 ml of TRI Reagent (Sigma-Aldrich, St. Luis, MO, USA) with protease inhibitors and homogenized (Homogenizer Workcenter T10 Basic ULTRA-TURRAX®, Whitestown, Ireland). Finally, a standard TRIzol protocol was applied for RNA and protein isolation.
Isolated total RNA was further purified using a TURBO DNA-free kit (Thermo Scientific, Waltham, MA, USA). SuperScript IV (Invitrogen, Carlsbad, CA, USA) was then used to transcribe the mRNA to cDNA. The level of cDNA synthesis was evaluated by real-time PCR as described above. Based on the available literature, three reference genes were selected, including Ywhaz, Hmbs, and Pabp (16). The qRT-PCR sequence primers are listed in Table 2.
Statistical analysis
For the real-time PCR analysis, the experimental samples were run in duplicate. Values are shown as the mean ± SEM. Statistical significance was evaluated with U Mann-Whitney and ANOVA tests. Values with P < 0.05 were considered to be statistically significant.
RESULTS
Transfection efficiency of human SkMDS/PCs with the pCiNeo-GJA1 plasmid
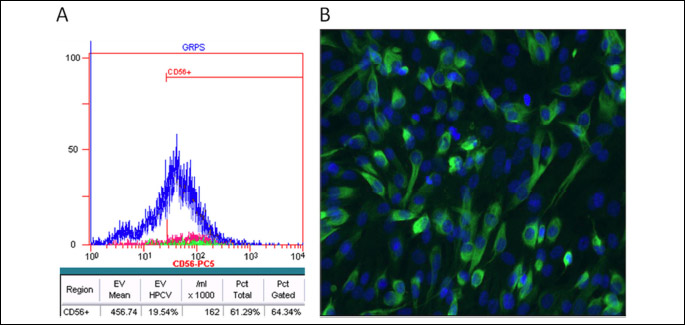
A-flow cytometry analysis of CD56+ expression on human SkMDS/PCs revealed that over 65% of cells were positive for the CD56+ marker (Fig. 3A) while desmin was confirmed in about 95% skeletal muscle-derived stem/progenitor cells used in this study (Fig. 3B). To evaluate the transfection efficiency of SkMDS/PCs with the pCiNeo-GJA1 plasmid, we stained pCiNeo-GJA1-transfected SkMDS/PCs with an anti-connexin-43 antibody. The transfection efficiency was approximately 96%. Figure 4 shows representative images of human SkMDS/PCs cells after transfection with the pCiNeo-GJA1 plasmid; a clear difference between ‘native’ and connexin-43-overexpressing SkMDS/PCs can be seen in these images. SkMDS/PCsWT showed rather weak staining, whereas SkMDS/PCsCX43 were characterized by a significant amount of connexin-43 protein, as expected.
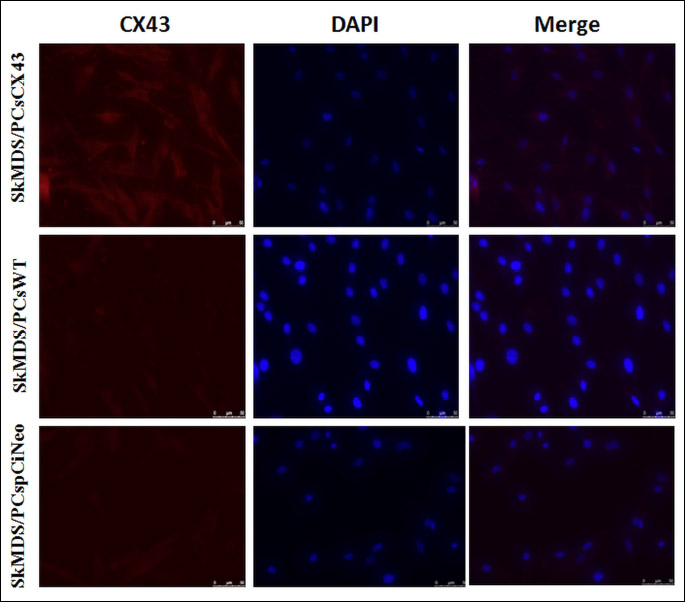
Abbreviations: CX43, connexin-43 protein; DAPI, 4',6-diamidyno-2-fenyloindol (nuclear marker); SkMDS/PCsCX43, skeletal muscle-derived stem/progenitor cells transfected with the pCiNeo-GJA1 plasmid; SkMDS/PCspCiNeo, Mock-transfected skeletal muscle-derived stem/progenitor cells; SkMDS/PCsWT, Control, wild-type skeletal muscle-derived stem/progenitor cells.
Impact of transient SkMDS/PCs transfection on the amount of connexin-43 expression in genetically modified human skeletal muscle-derived stem/progenitor cells
The expression level of gene encoding connexin-43 was approximately 40-fold higher at the mRNA level in the SkMDS/PCs population transfected with pCiNeo-GJA1 compared to the SkMDS/PCs population transfected with pCiNeo (Fig. 5A). In addition, at the protein level, we observed an increased amount of connexin-43 (4-fold) in the SkMDS/PCs population transfected with the pCiNeo-GJA1 plasmid (Fig. 5B) compared to the SkMDS/PCs population transfected with pCiNeo alone (SkMDS/PCspCiNeo).
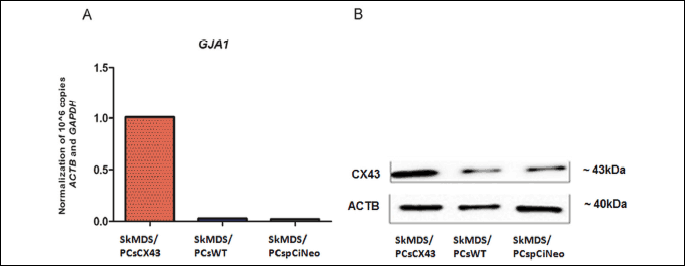
Abbreviations: ACTB, actin beta protein; CX43, connexin-43 protein; SkMDS/PCsCX43, skeletal muscle-derived stem/progenitor cells transfected with the pCiNeo-GJA1 plasmid; SkMDS/PCspCiNeo, Mock-transfected skeletal muscle-derived stem/progenitor cells; SkMDS/PCsWT, Control, wild-type skeletal muscle-derived stem/progenitor cells.
Transient SkMDS/PCs transfection specifically extends the cell proliferation stage
Fig. 6 shows the results with respect to the genetically modified SkMDS/PCs. We observed a statistically significant downregulation of three myogenic genes, including myosin heavy chain 1 (MYHC1), myogenin (MYOG) and myogenic differentiation 1 (MYOD). It should be noted that similar trends were also observed for MYHC1 and MYOG after transfection with the empty plasmid. The slightly increased expression levels of MYF5 (Fig. 6A) were not statistically significant.
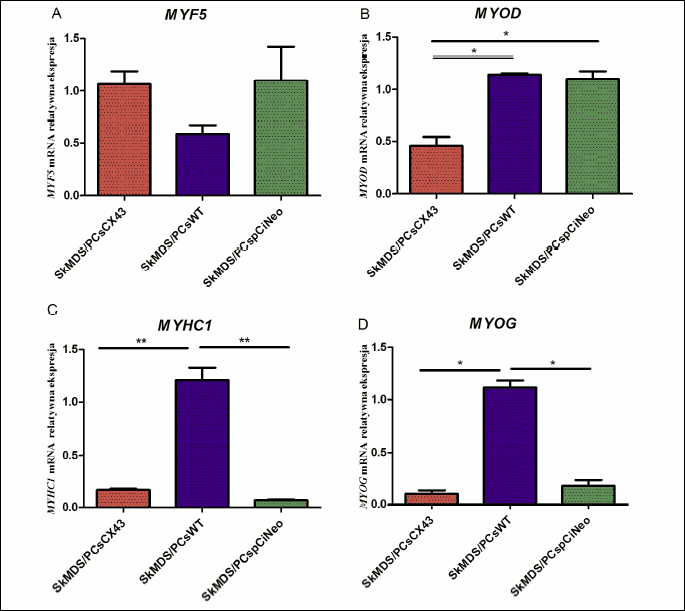
Abbreviations: SkMDS/PCsCX43, skeletal muscle-derived stem/progenitor cells transfected with the pCiNeo-GJA1 plasmid; SkMDS/PCspCiNeo, Mock-transfected skeletal muscle-derived stem/progenitor cells; SkMDS/PCsWT, Control, wild-type skeletal muscle-derived stem/progenitor cells. Gene abbreviations: MYF5, myogenic factor 5; MYOD, myogenic differentiation 1; MYHC1, myosin heavy chain 1; MYOG, myogenin.
SkMDS/PCs overexpressing connexin-43 align well with host cardiomyocytes
Fig. 7A shows the results of antibody-specific staining directed against human nuclei membranes (brown staining) and a control statining, performed in MI + 0.9% NaCl group (Fig. 7B). Moreover, a typical staining for connexin-43 protein was also observed (Fig. 8A1-8A3 black arrows), as were anti-alpha smooth muscle actin antibodies, both of which labelled human myotubes (Fig. 8B1-8B3). The latter staining against SMA also showed that implanted SkMDS/PCs in the MI + SkMDS/PCsWT group (Fig. 8B2) were randomly scattered, while SkMDS/PCs overexpressing connexin-43 were aligned in the typical sarcomeric structures formed by cardiomyocytes (Fig. 8B3).
The results obtained from immunohistochemical staining using these antibodies provide unequivocal evidence for the presence of implanted human SkMDS/PCs in rat post-infarcted hearts at four weeks after cell intervention (Figs. 7 and 8).
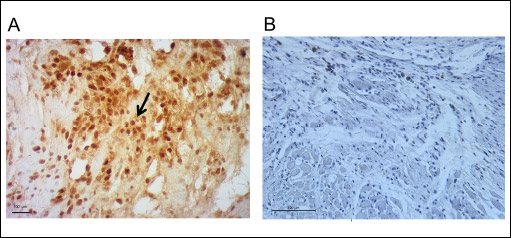 |
Fig. 7. Anti-human nucleus (MAB 1281) staining results. (Panel A): The photograph shows visibly stained brown human cell nuclei, which is the evidence for the presence of human SkMDS/PCs in the post-infarcted rat hearts at 4 weeks after the cell intervention (arrows). (Panel B): The negative immunohistochemical staining results after MI + 0.9% NaCl intervention. Control staining included isotype of mouse monoclonal antibody mix from Dako/Perlan. |
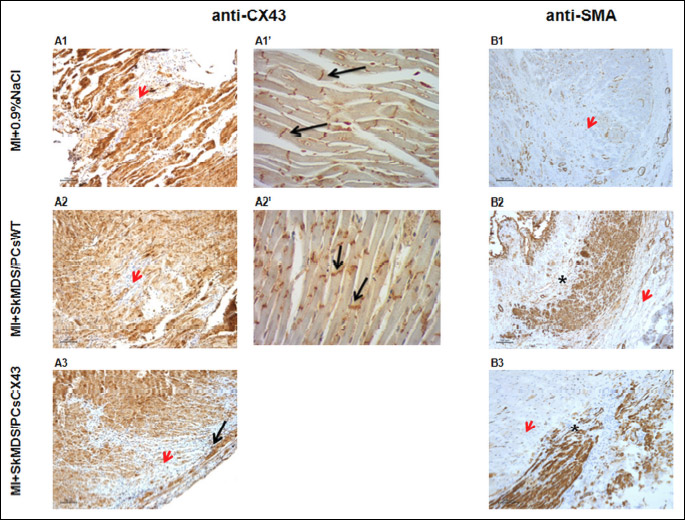
Abbreviations: MI + 0.9%NaCl, animals in which myocardial infarction was induced and a 0.9% solution of physiological saline was delivered (‘sham’ intervention); MI + SkMDS/PCsWT, animals in which myocardial infarction was induced and control non-transfected SkMDS/PCs were administered; MI + SkMDS/PCsCX43, animals in which myocardial infarction was induced and SkMDS/PCs overexpressing connexin-43 were administered; anti-CX43, antibody against connexin-43; anti-SMA, antibody against smooth muscle actin.
Anti-arrhythmic effect of genetically modified SkMDS/PCs
Infarct sizes induced in rat groups under study were comparable and did not reveal statistically significant differences (Fig. 9). The connexin-43-modified SkMDS/PCs were shown to significantly reduce the occurrence of additional secondary ventricle stimulations in post-infarcted rat hearts compared to those of the group treated with ‘native’ SkMDS/PCs. This effect was most clearly observed at one month after the cell interventions while sham intervention group of rats did not reveal any arrhythmic episodes (Fig. 10). The results obtained after the induction of ventricular arrhythmia in rats were scored (Table 3) using the relevant descriptions of arrhythmia listed in Table 4.
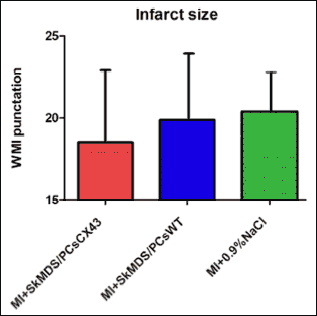 |
Fig. 9. Myocardial infarct sizes identified in the left heart ventricles of the rats. No significant differences were observed for the groups tested. The left ventricle WMI index was applied to assess the infarct size. Abbreviations: MI + SkMDS/PCsCX43, animals in which myocardial infarction was induced and SkMDS/PCs overexpressing connexin-43 were administered; MI + SkMDS/PCsWT, animals in which myocardial infarction was induced and control non-transfected SkMDS/PCs were administered; MI + 0.9%NaCl, animals in which myocardial infarction was induced and a 0.9% solution of physiological saline was delivered (‘sham’ intervention); WMI, wall motion score index. |
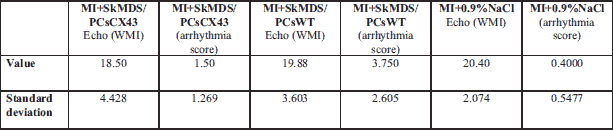
Abbreviations: Echo, echocardiography; MI, myocardial infarction; WMI, wall motion index; MI + SkMDS/PCsCX43, animals in which myocardial infarction was induced and SkMDS/PCs overexpressing connexin-43 were administered; MI + SkMDS/PCsWT, animals in which myocardial infarction was induced and control non-transfected SkMDS/PCs were administered; MI + 0.9%NaCl, animals in which myocardial infarction was induced and a 0.9% solution of physiological saline was delivered (‘sham’ intervention).
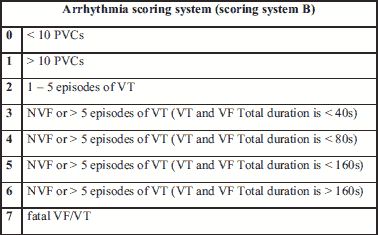
Genetically modified SkMDS/PCs with gene encoding CX43 affect the expression of selected genes in myocardium
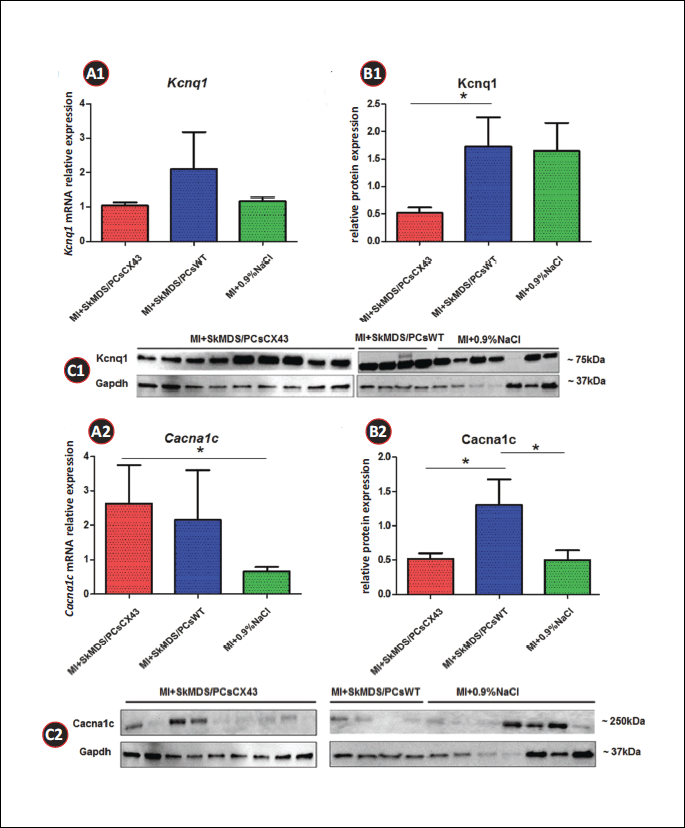
We examined the levels of genes involved in heart conductivity, Kcnq1, Cacna1c, and Ncx1, as well as those of genes responsible for heart remodelling, Serca2a, Tgfb1, and Gata4. The genetic profile data obtained (Figs. 11A1, 11A2, 12A1, 12A2 and 13A1, 13A2) were generally not in accord with the results obtained from the in vivo functional studies (reduced cardiac arrhythmia in MI + SkMDS/PCsCX43 rats; Fig. 10). The animal subgroups treated with connexin-43-overexpressing SkMDS/PCs had significantly increased Cacna1c expression and significantly decreased Serca2a expression, opposite to the expected results. However, mRNA expression did not necessarily match the observed protein expression, as is also seen in Figs. 11B1, 11B2, 12B1, 12B2 and 13B1, 13B2. In mammalian cells, the correlation between mRNA and protein expression has been shown to be weak and to be dependent on activity and the cell type. The half-life of various types of proteins can range from minutes to days, with up to 48 hours required to protein degradation, while mammalian mRNA transcript degradation rates can range from 2 – 7 hours (17). It should be emphasized that the functionally critical effector molecules governing the cell function are proteins and not mRNA transcripts.
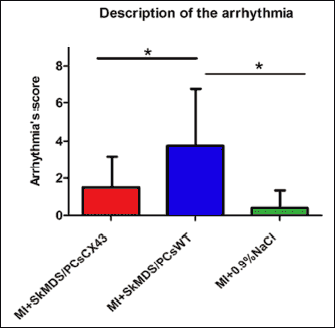 |
Fig. 10. Average arrhythmia score for each rat subgroup studied. The arrhythmia index was calculated based on the arrhythmia scoring system presented in Table 4. Values are presented as the mean ± SE. *P < 0.05 and **P < 0.01. Abbreviations: MI + SkMDS/PCsCX43, animals in which myocardial infarction was induced and SkMDS/PCs overexpressing connexin-43 were administered; MI + SkMDS/PCsWT, animals in which myocardial infarction was induced and control non-transfected SkMDS/PCs were administered; MI + 0.9% NaCl, Animals in which myocardial infarction was induced and a 0.9% solution of physiological saline was delivered (‘sham’ intervention). |
Connexin-43-overexpressing SkMDS/PCs exert an anti-arrhythmic effect
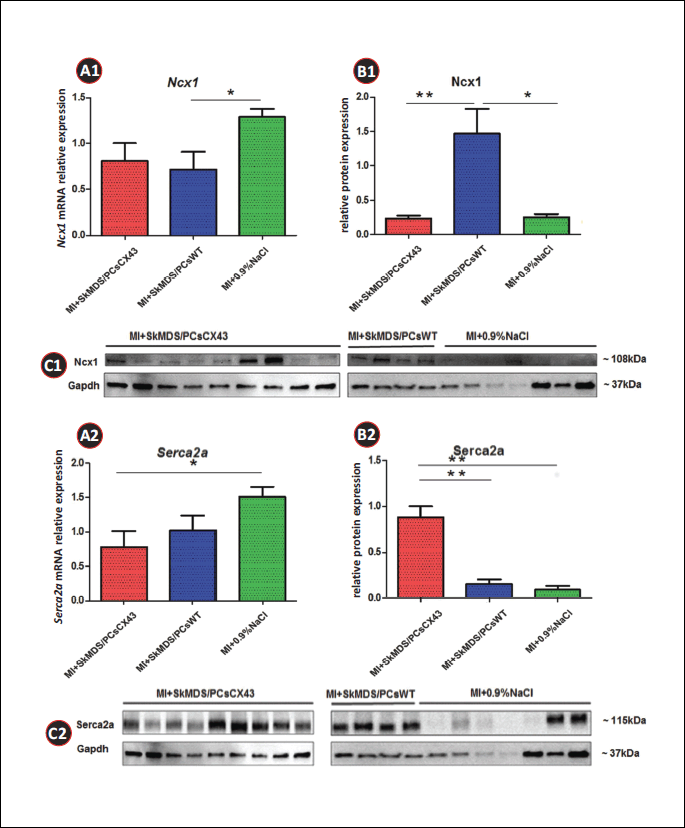
The results obtained indicate that after SkMDS/PCsCX43 intervention in rats, the protein expression levels of Cacna1c and Ncx1 decreased and were closer to those of the control ‘sham’ group (MI + 0.9%NaCl). Another analysed protein, Serca2a, was significantly upregulated, which could indicate the anti-arrhythmic protection of the implanted cells overexpressing connexin-43. In the Western blot analysis for Tgfb1, we used four different antibodies, but no positive results were obtained. This finding may suggest a lack of translated protein in the myocardium at the time points studied. These data have been graphically illustrated in Figs. 11B1, 11B2, 12B1, 12B2 and 13B1, 13B2.
Gene abbreviations: Kcnq1, potassium voltage-gated channel subfamily Q member 1; Cacna1c, calcium voltage-gated channel subunit alpha1 C; Hmbs, hydroxymethylbilane synthase; Pabp, poly(A)-binding protein; Ywhaz, tyrosine 3-monooxygenase.
Abbreviations: MI + SkMDS/PCsCX43, Animals in which myocardial infarction was induced and SkMDS/PCs overexpressing connexin-43 were administered; MI + SkMDS/PCsWT, animals in which myocardial infarction was induced and control non-transfected SkMDS/PCs were administered; MI + 0.9%NaCl, animals in which myocardial infarction was induced and a 0.9% solution of physiological saline was delivered (‘sham’ intervention).
DISCUSSION
Myocardial infarction is the leading cause of death in people around the world. Currently, no standard treatment method is able to generate new contractile tissue or restore the functional environment of infarcted myocardium. Therefore the transplantation of skeletal muscle stem cells can be a promising strategy for the treatment of heart failure. Our previous observations also provided positive conclusions that myoblasts can be used for the regeneration of ischemic heart. Furthermore, we have shown that the hypoxic conditions prevailing in the post-infarcted heart can provide an environment for high expression of prioangiogenic genes, i.e. potentially improving new blood vessel formation in the post infarction area (18).
However, the human myoblast transplants currently used for cardiac repair comprise a non-optimized therapeutic modality (19-21). Patients in the many clinical trials testing the application of myoblasts in post-infarcted hearts have been plagued by arrhythmias; this outcome can be attributed to the electrical isolation of human myoblasts due to the absence of ‘gap junctions’, leaving them disconnected from the recipient organ cardiomyocytes (22).
The main aim of this work was to examine whether human skeletal muscle-derived stem/progenitor cells overexpressing connexin-43 can improve intercellular communication, thus reducing arrhythmogenic phenomena after their transplantation into the post-infarcted myocardium.
To inhibit arrhythmia in an infarcted heart, clarifying the underlying molecular mechanisms and identifying effective therapeutic targets are crucial. Potentially lethal ventricular arrhythmias, such as ventricular tachycardia or ventricular fibrillation, usually occur when a trigger (most often a ventricular extrasystolic beat) encounters favourable electrophysiological conditions. Ventricular extrasystolic beats are triggered by early afterdepolarizations (EADs) that occur during the plateau phase or during repolarization of the action potential and by delayed afterdepolarizations (DADs) that occur after completion of the action potential. Abnormal intracellular Ca2+ handling, including sarcoplasmatic reticulum (SR) overload, uncontrolled Ca2+ leakage from SR calcium channels (ryanodine receptors (RyRs)), increased Na+/Ca2+ exchange and reduced Serca2a expression, are the main source of DAD (23).
Conversely, EAD results from increased afterdepolarizing potential (ADP), which is caused by reduced potassium channel function or increased calcium or sodium channel activity. Furthermore, heterogeneous prolongation of APD in adjacent groups of cells (dispersion of repolarization) can result in a unidirectional block, initiating re-entry. Moreover, re-entry is favoured by slow conductivity, which can be caused by a reduced action potential (AP) amplitude, a reduction of intercellular connections (so called connexons) and increased myocardial fibrosis (24). In summary, the main factors that favour life-threatening arrhythmias include: 1) abnormal intracellular Ca2+ handling, 2) changes in the action potential duration (particularly when they are heterogeneous), 3) extracellular matrix remodelling that results in excessive fibrosis and 4) reduced intercellular connections.
We found that after the implantation of genetically modified SkMDS/PCs in left ventricle (LV) tissue, the expression of Ncx1 is decreased at the protein level (Fig. 12B1), while that of Serca2a is increased (Fig. 12B2) (i.e., the ratio of Ncx1/Serca2a is significantly decreased (Fig. 13B)). This finding is very important in the context of the arrhythmic propensity of the heart and suggests that more Ca2+ is transported into the sarcoplasmic reticulum and less is transported through the cell membrane by Ncx1. In its normal mode, Ncx1 transports 1 Ca2+ ion out of the cell and 3 Na+ ions into the cell, which is accompanied by a depolarizing current, the main source of DAD. Decreased Ncx1 expression accompanied by increased Serca2a expression lowers the possibility of DAD induction. Moreover, we have shown that the expression of Kcnq1 at the protein level was decreased, which could prolong APD and promote EADs. However, at the same time, Cacna1c expression was decreased after MI + SkMDS/PCsCX43 intervention, possibly counteracting potassium channel-dependent AP. The net effect would be no change in the propensity of EAD induction.
Gene abbreviations: Ncx1, sodium/calcium exchanger protein; Serca2a, ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2; Hmbs, hydroxymethylbilane synthase; Pabp, poly(A)-binding protein; Ywhaz, tyrosine 3-monooxygenase.
Abbreviations: MI + SkMDS/PCsCX43, animals in which myocardial infarction was induced and SkMDS/PCs overexpressing connexin-43 were administered; MI + SkMDS/PCsWT, animals in which myocardial infarction was induced and control non-transfected SkMDS/PCs were administered; MI + 0.9%NaCl, animals in which myocardial infarction was induced and a 0.9% solution of physiological saline was delivered (‘sham’ intervention).
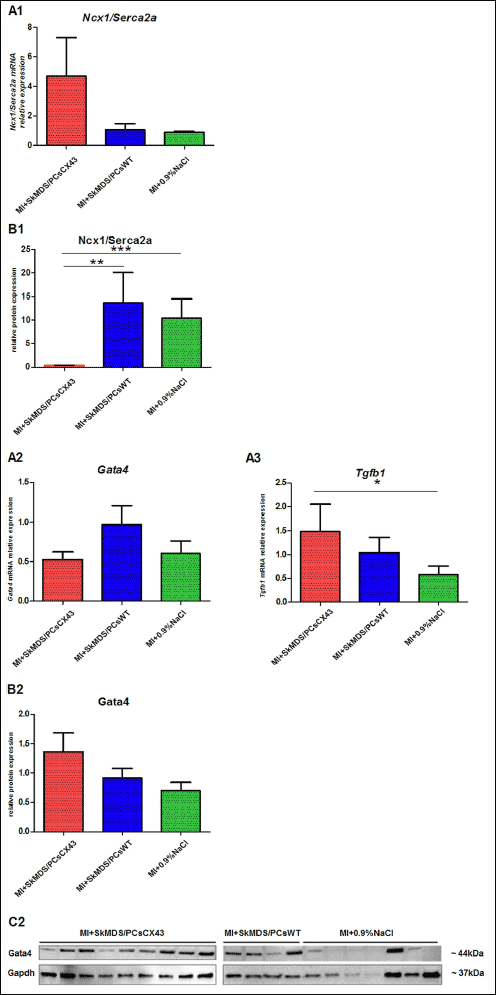 |
Fig. 13. The long-term effects of the cell interventions on Ncx1/Serca2a ratio, Gata4 and Tgfb1 expression in the myocardium at 42 days after MI induction. The expression levels of selected genes were normalized to three housekeeping genes (Ywhaz, Hmbs, and Pabp). Panel A shows the gene expression with respect to the protein product presented in Panel B, which was determined with respect to the housekeeping genes and their products. Panel C shows the detection of selected proteins (Western immunoblotting) in the heart tissue samples. Values are given as the mean ± SD. *P < 0.05, **p < 0.01, and ***P < 0.001. Gene abbreviations: Ncx1/Serca2a, ratio of Ncx1 to Serca2A; Gata4, GATA binding protein 4; Tgfb1, transforming growth factor beta 1; Hmbs, hydroxymethylbilane synthase; Pabp, poly(A)-binding protein; Ywhaz, tyrosine 3-monooxygenase. Abbreviations: MI + SkMDS/PCsCX43, animals in which myocardial infarction was induced and SkMDS/PCs overexpressing connexin-43 were administered; MI + SkMDS/PCsWT, animals in which myocardial infarction was induced and control non-transfected SkMDS/PCs were administered; MI + 0.9%NaCl, Animals in which myocardial infarction was induced and a 0.9% solution of physiological saline was delivered (‘sham’ intervention). |
Moreover, a change in connexin-43 distribution may be observed in post-infarction heart and can be related to inflammation provoked by ischemia. Such observation was made by another group which applied a bolus of bacterial lipopolysaccharide (LPS) to the spontaneously hypertensive rats (SHR) and age-matched normotensive Wistar rats. They observed changes in spatial expression of connexin-43 in the heart which may be associated with increased macrophage infiltration due to existing inflammation in the post-infarction myocardium (25).
Perhaps, the increased expression of connexin-43 in transplanted SkMDS/PCs contributes to the reduction of inflammation of the in the peri-infarcted region thus causing an indirect positive effect on calcium intracellular circulation.
We may assume that, connexin-43-modified human SkMDS/PCs, which properly aligned in heart tissue and electrically coupled with each other and with neighbouring cardiomyocytes, reduced the intercellular conduction velocity to a much lesser degree than that of the diffuse, electrically uncoupled native SkMDS/PCs. Better intracellular conduction means a smaller risk of inducting and maintaining re-entry arrhythmias.
One of our objectives was to obtain a suspension of human SkMDS/PCs overexpressing the DNA sequence encoding connexin-43 and to verify its overexpression at both the mRNA and protein levels. The transfection efficiency, which was assessed to be approximately 96%, was examined using a specific antibody against human connexin-43. We demonstrated 40-fold higher mRNA expression levels and 4-fold higher protein levels compared to that of the non-transfected and wild-type control cells, respectively. Using a rat model of post-infarction heart we showed that the transplantation of SkMDS/PCs overexpressing connexin-43 prevented the development of spontaneous ventricular tachycardias (VTs) (Fig. 10). In contrast, the level of cardiac arrhythmias in the MI+SkMDS/PCsWT rat intervention group was significantly elevated while physiological saline was not arrhythmogenic, so arrhythmia was not observed in the MI + 0.9% NaCl treated group.
It should be noted that connexin-43 expression was observed to influence the expression of genes involved in myogenesis (Fig. 6). These genes can be subdivided according to the sequence of myogenic events and the involved transcription factors that govern this process. As can be inferred from Fig. 6, upon GJA1 transfection, the transcription factor that initializes cell activation (Fig. 6A-6B) was in the upper expression range (MYF5), while the genes responsible for terminal myogenic differentiation were clearly downregulated (Fig. 6C-6D). Moreover, transfection with an empty pCiNeo vector exerted a similar effect on the MYOG and MYHC1 genes. Electroporation itself may have had a positive effect on the expression of these two genes; however, these observations can be treated as a beneficial phenomenon because the decreased expression of MYOG and MYHC1 indicates a specific extension of the cell proliferation stage, which slows down the process of differentiation into myotubes.
Collectively, cell transplantation can be assumed to be more effective in the absence of SkMDS/PCs necrosis in the infarction area because the SkMDS/PCs efficiently proliferate and produce the connexin-43 protein before they are finally differentiated; this occurrence is necessary for the formation of intercellular links between the implanted cells and the recipient cells and contributes to better ion flow, consequently reducing cardiac arrhythmia after myocardial infarction.
Alfa-smooth muscle actin is a protein typically present in primary human myoblasts, myofibroblasts and vascular smooth muscle cells. Springer et al. observed that SMA expression appeared during the initial elongation of the cell and persisted through early period of differentiation into myotubes (26). Therefore in our view anti-SMA staining supports the evidence of the presence of SkMDS/PCs in myocardium. Our immunostaining results (Fig. 8B1-8B3) demonstrate that SMA protein expression may occurr in myotubes differentiated from connexin-43-overexpressing human SkMDS/PCs, and judging upon its morphology did not correspond in any way to the myofibroblasts or vascular smooth muscle cells. Moreover, in Fig. 7B3, a striated pattern that is characteristic of sarcomeric structures of skeletal muscles has been apparent.
We shall emphasize that, for the first time, we have demonstrated that both types of transplanted SkMDS/PCs (‘native’ and connexin-43-modified) were present in the myocardium at one month after cell administration (Fig. 7 and 8A1-8A3). However, the implanted ‘native’ SkMDS/PCs were scattered, while SkMDS/PCs overexpressing connexin-43 were aligned in typical structures formed by cardiomyocytes (Fig. 8B2-8B3). We believe that due to the overexpression of connexin-43, the SkMDS/PCsCX43 easily connected with each other and with surrounding cardiomyocytes, forming a functional syncytium and improving LV contractile function. Unfortunately, we were unable to evaluate LV function by echocardiography in this study, but our supposition is conceivable given our immunohistochemical staining results.
In our study, we investigated the expression of the main structural elements of the potassium (Kcnq1), calcium (Serca2a, Ncx1, and Cacna1c) and calcium/sodium channels (Ncx1) in the heart at the gene and protein levels. To compare the expression of a particular gene with its respective protein product, RNA and protein isolations were performed using the same analysed tissue sample, each of which represented the whole left ventricle of a rat heart.
Figs. 11-13 summarize the gene and protein expression levels of all the tested ion channels. It should be noted that the applied cell interventions (SkMDS/PCsWT or SkMDS/PCsCX43) caused some changes in gene expression at the mRNA and protein levels; these changes were long-lasting and were present in the left ventricles subjected to cell interventions compared to the ‘sham’ treatment. Generally, the genes encoding these channels were downregulated after the administration of genetically modified SkMDS/PCs compared to the SkMDS/PCsWT intervention, with the exception of Cacna1c (transcription). A visible neutral effect was observed for Gata-4 gene expression, while Tgfb1 gene transcription was upregulated. On the contrary, protein expression of respective genes were more favourable to maintain antiarrhythmic environment.
Diastolic wall stress is the main trigger of cardiac remodelling both at the cellular and molecular levels. Increased contractility due to the application of Cardiac Contractility Modulation (CCM) therapy has been shown to induce changes in gene expression for genes involved in the regulation of calcium homeostasis and arrhythmogenesis (i.e., Ncx1, Serca2a, and RyRs) as early as 2 hours after modulating signal onset in the region of the electrodes. After 3 months of CCM treatment, both local and remote sites demonstrated similar improvement at the protein level (27).
Moreover, wall stress is the main regulator of B-type natriuretic peptide (BNP) synthesis in ventricular cardiomyocytes. Serca2a expression is negatively regulated by BNP, probably through a Ca2+-dependent pathway (28). Connexin-43-modified SkMDS/PCs possibly increase the contractility of the LV myocardium and decrease wall stress and BNP synthesis (supported by our earlier observation; (14)), ultimately leading to increased Serca2a expression, which we found in the MI + SkMDS/PCsCX43 intervention group. In addition, increased Serca2a protein expression can result in increased SR Ca2+ content and Ca2+ transient amplitudes as well as cell shortening. The increased contractile force of individual cardiomyocytes improves LV function and further decreases diastolic wall stress, which may promote gene expression regulation.
In this study, we did not investigate a hemodynamics of the myocardium, since this was not a primary goal of the conducted experiments. However, in our previous reports we have examined the changes in the heart functional parameters using echocardiography prior to and after the cell transplantation to assess the effect of cell intervention on the myocardium of post-infarction heart (11). We observed that after transplantation of genetically modified SkMDS/PCs, the left ventricular area in the short axis (SAX AC%) was not significantly altered after the two months of the experiment. On the contrary to the spontaneous remodelling progression in untreated post-infarction heart, in which a concomitant significant decrease in the measured ejection fraction was observed (P < 0.01) (11). The other study in a rat model also documented beneficial effects of implanted myoblasts on heart hemodynamics (29).
In summary, we hypothesize that connexin-43-enriched SkMDS/PCs better integrate into a functional cardiomyocyte syncytium than native SkMDS/PCs, improving contractile function, decreasing wall stress, promoting changes in arrhythmogenic gene expression and in a long term reducing pro-arrhythmic susceptibility in the failing heart (positive protein product regulation).
In conclusion, the beneficial implemented genetic modification of SkMDS/PCs was confirmed in both in vivo and ex vivo systems. In the in vivo system, we showed that application of genetically modified SkMDS/PCs (with the transiently expressed pCiNeo-GJA1 plasmid) can exert therapeutic effect on post-infarction rat at the same time, decreasing the occurrence of the spontaneous and induced ventricular arrhythmias compared to ‘native’ SkMDS/PCs.
Moreover, in the ex vivo system, gene and protein expression analysis of structural ion channels showed that connexin-43-overexpressing SkMDS/PCs displayed anti-arrhythmic characteristics that positively impacted the duration of action potentials in the monitored myocardium (calcium ions). We also observed a statistically significant increase in Serca2a protein expression in the MI + SkMDS/PCsCX43 group compared to MI + SkMDS/PCsWT group and MI + 0.9%NaCl control interventions. These results provide an additional proof of the beneficial therapeutic characteristics of SkMDS/PCsCX43 cells and indicates that Serca2a can be important validation biomarker indicating the successful prevention of myocardial remodelling.
Authors’ contributions: A. Rugowska performed and designed the majority of experiments and drafted the first version of the manuscript; B. Wiernicki performed the animal experiments; M. Maczewski performed the arrhythmia induction experiments; U. Mackiewicz interpreted the pro-arrhythmia data; K. Chojnacka and A. Kluk performed the immunohistochemical staining; K. Bednarek-Rajewska observed the staining and interpreted the histopathological results; P. Majewski supervised and verified the histopathological results; T. Kolanowski. assisted with animal preparation for the post-infarction model; A. Malcher designed the protocols for DNA, RNA and protein extraction; N. Rozwadowska assisted with the study design and data interpretation; and M. Kurpisz supervised the study, provided financial support and finalized the manuscript.
Acknowledgements: We are grateful to Dr Agnieszka Zimna (Institute of Human Genetics, Polish Academy of Sciences, Poznan, Poland) for help with the in vitro cell culture. This work was supported by the National Science Centre, Poland grant no. 2012/07/N/NZ4/02117, and by the National Centre for Research and Development, Poland grant no. PBS3/A7/27/2015 and grant no. STRATEGMED1/233624/5/NCBR/2014.
Conflict of interest: None declared.
REFERENCES
- Hasan A, Khattab A, Islam MA, et al. Injectable hydrogels for cardiac tissue repair after myocardial infarction. Adv Sci (Weinh) 2015; 2: 1500122. doi:10.1002/advs.201500122
- Ghostine S, Carrion C, Souza LC, et al. Long-term efficacy of myoblast transplantation on regional structure and function after myocardial infarction. Circulation 2002; 106 (Suppl. 1): I131-I136.
- Horackova M, Arora R, Chen J, et al. Cell transplantation for treatment of acute myocardial infarction: unique capacity for repair by skeletal muscle satellite cells. Am J Physiol Heart Circ Physiol 2004; 287: H1599-H1608.
- Siminiak T, Kalawski R, Fiszer D, et al. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: Phase I clinical study with 12 months of follow-up. Am Heart J 2004; 148: 531-537.
- Siminiak T, Fiszer D , Jerzykowska O, et al. Percutaneous trans-coronary-venous transplantation of autologous skeletal myoblasts in the treatment of post-infarction myocardial contractility impairment: the POZNAN trial. Eur Heart J 2005; 26: 1188-1195.
- Miyagawa S, Sawa Y. From bench to bedside , work in cell-based myocardial regeneration therapy. J Biomed Sci Engineering 2014; 7: 86-103.
- Menasche P. Stem cell therapy for heart failure: are arrhythmias a real safety concern? Circulation 2009; 119: 2735-2740.
- Reinecke H, MacDonald GH, Hauschka SD, Murry CE. Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair. J Cell Biol 2000; 149: 731-740.
- Balogh S, Naus CC, Merrifield, PA. Expression of gap junctions in cultured rat L6 cells during myogenesis. Dev Biol 1993; 155: 351-360.
- Formigli L, Francini F, Tani A, et al. Morphofunctional integration between skeletal myoblasts and adult cardiomyocytes in coculture is favored by direct cell-cell contacts and relaxin treatment. Am J Physiol Cell Physiol 2005; 288: 795-804.
- Kolanowski TJ, Rozwadowska N, Malcher A, et al. in vitro and in vivo characteristics of connexin 43-modified human skeletal myoblasts as candidates for prospective stem cell therapy for the failing heart. Int J Cardiol 2014; 173: 55-64.
- Rozwadowska N, Fiszer D, Siminiak T, et al. Evaluation of in vitro culture of human myoblasts for tissue autotransplants to the post-infarcted heart. Pol Heart J 2002; 57: 233-237.
- Bialas M, Krupka M, Janeczek A, et al. Transient and stable transfection of mouse myoblasts with genes coding for pro-angiogenic factors. J Physiol Pharmacol 2011; 62: 219-228.
- Wiernicki B, Rozwadowska N, Malcher A, et al. Human myoblast transplantation in mice infarcted heart alters the expression profile of cardiac genes associated with left ventricle remodeling. Int J Cardiol 2016; 202: 710-721.
- Hummon AB, Lim SR, Difilippantonio MJ, Ried T. Isolation and solubilization of proteins after TRIzol® extraction of RNA and DNA from patient material following prolonged storage. Biotechniques 2007; 42: 467-472.
- De Spiegelaere W, Dern-Wieloch J, Weigel R, et al. Reference gene validation for RT-qPCR, a note on different available software packages. PLoS One 2015; 10: e0122515. doi: 10.1371/journal.pone.0122515
- Vogel C, Marcotte EM. Insights into regulation of protein abundance from proteomics and transcriptomis analyses. Nat Rev Genet 2012; 13: 227-232.
- Zimna A, Wiernicki B, Kolanowski T, et al. Influence of hypoxia prevailing in post-infarction heart on proangiogenic gene expression and biological features of human myoblast cells applied as a pro-regenerative therapeutic tool. J Physiol Pharmacol 2018; 69: 859-874.
- Homma J, Sekine H, Matsuura K, Yamato M, Shimizu T. Myoblast cell sheet transplantation enhances the endogenous regenerative abilities of infant hearts in rats with myocardial infarction. J Tissue Eng Regen Med 2017; 11: 1897-1906.
- Povsic TJ, O'Connor CM, Henry TA, et al. A double-blind, randomized, controlled, multicenter study to assess the safety and cardiovascular effects of skeletal myoblast implantation by catheter delivery in patients with chronic heart failure after myocardial infarction. Am Heart J 2011; 162: 654-662. e1.
- Terajima Y, Shimizu T, Tsuruyama S, et al. Autologous skeletal myoblast sheet therapy for porcine myocardial infarction without increasing risk of arrhythmia. Cell Med 2013; 6: 99-109.
- Wollert KC, Meyer GP. Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The BOOST randomised controlled clinical trial. Lancet 2004; 364: 141-148.
- Antzelevitch C, Burashnikov A. Overview of basic mechanisms of cardiac arrhythmia. Card Electrophysiol Clin 2011; 3: 23-45.
- Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 2007; 87: 425-456.
- Okruhlicova L, Cicakova Z, Frimmel K, et al. Lipopolysaccharide-induced redistribution of myocardial connexin43 is associated with increased macrophage infiltration in both normotensive and spontaneously hypertensive rats. J Physiol Pharmacol 2018; 69: 709-717.
- Springer ML, Ozawa CR, Blau HM. Transient production of alfa-smooth muscle actin by skeletal myoblasts during differentiation in culture and following intramuscular implantation. Cell Motil Cytoskeleton 2002; 51: 177-186.
- Butter C, Rastogi S, Minden HH, Meyhofer J, Burkhoff D, Sabbah HN. Cardiac contractility modulation electrical signals improve myocardial gene expression in patients with heart failure. J Am Coll Cardiol 2008; 51: 1784-1789.
- Toischer K, Kogler H, Tenderich G, et al. Elevated afterload, neuroendocrine stimulation, and human heart failure increase BNP levels and inhibit preload-dependent SERCA upregulation. Circ Heart Fail 2008; 1: 265-271.
- Giraud MN, Liechti EF, Tchantchaleishvili V, et al. Myocardial injection of skeletal myoblasts impairs contractility of host cardiomyocytes. Int J Cardiol 2010; 138: 131-137.
A c c e p t e d : December 30, 2019