ALTERATIONS IN GLUTATHIONE PEROXIDASE AND SUPEROXIDE DISMUTASE ACTIVITIES IN PLASMA AND SALIVA IN RELATION TO DISEASE ACTIVITY IN PATIENTS WITH CROHN'S DISEASE
INTRODUCTION
Crohn's disease (CD) is a chronic inflammatory disorder of the entire gastrointestinal (GI) tract, which most commonly affects the terminal ileum and proximal colon (1-3). In approximately half of patients the course of the disease is complicated by the formation of fistulas, intra-abdominal abscesses, and intestinal strictures (1, 2). The precise etiopathogenesis of CD remains unknown. Interactions among various factors, including genetics, the host immune system, microbiome, and environmental factors play an important role in disturbing the intestinal homeostasis, leading to the dysregulated inflammatory response of the GI tract (1, 2, 4-8). Different types of cytokines are involved in this process with predominance of tumour necrosis factor-alpha, which plasma level is elevated in about 50% of patients with CD (9). The hyper-reactive immune system is accompanied by the presence of oxidative stress (OxS) with increased release of reactive oxygen species (ROS). ROS are modulated in normal conditions by different elements, including enzymes of the antioxidant defence, which prevent the harmful effects of ROS on the tissue (7, 10). Many authors have shown that CD is associated with an imbalance between increased ROS and decreased antioxidant activity, resulting in OxS phenomena (7, 11-15). Currently, OxS is considered as a potential etiological factor for CD rather than a concomitant effect in the disease (7, 14). The persistence of OxS can also affect the course of the disease. Furthermore, the epigenetic mechanisms, mainly microRNAs, are considered key elements in the pathogenesis of CD (7, 14).
OxS has been proposed as one of the major contributing factors in the destruction of the GI tract, and this is associated with some of the characteristic features of CD, e.g. GI transmural inflammation and complications (12, 16). Therefore, it is important to better understand the pathophysiology of CD based on molecular aspects of OxS, because new antioxidant compounds like drugs, hormones, functional foods, probiotics, natural active compounds from vegetal sources could become promising therapeutic modalities of the disease (17-20).
Several authors have demonstrated that CD patients have decreased levels of antioxidative enzymes in the intestinal tissue (12, 16, 21-23), but the levels of the enzymes in plasma or serum depend on the activity of CD (6, 21). Only a few studies have been compared oxidant-antioxidant status in patients with active and inactive CD (5, 24). The main intracellular antioxidant enzymes participating in the protection of cells against the damaging effects of ROS are glutathione peroxidase (GPx) and superoxide dismutase (SOD) (6, 7).
Reduced activities of GPx, one of the most important antioxidant defence system in the body, were reported either increased or reduced in different tissues as intracellular antioxidant enzymes (25, 27). Several reports have shown a higher GSSG/GSH ratio in different fluids (27, 28), also in saliva of patients compared with healthy subjects (29-31). Patients with CD may present orofacial granulomatosis, as symptomatic oral disease, and other oral manifestation derived from qualitative changes in salivary composition (32). Elevated GPx activity was reported in the parotid glands of rats with experimental colitis, whereas reduced activity of the enzyme was found in submandibular glands (33).
The determination of the OxS may require invasive techniques such as blood samples. Exploration of saliva for oxidative stress markers that accurately reflect the redox status of the body may be of great clinical interest. Therefore, in this clinical study we compare GPx and SOD activity in plasma and saliva of patients with various phenotypes of CD.
The aim of the study was to assess the antioxidative stress enzymes, GPx and SOD, in the plasma and saliva of patients with active and inactive forms of CD, as well as to compare the activity of these enzymes with clinical features.
MATERIAL AND METHODS
Study population
Forty-seven patients with CD (mean age 32 ± 10.1 years; 28 males and 19 females) were prospectively enrolled in the study. Patients were recruited between December 2014 to November 2015 from the Division of Gastroenterology and Hepatology at the University Hospital in Cracow. The study was performed in accordance with the ethical principles of the Helsinki Declaration of 2008. Informed consent to the study procedure was obtained from all participants. The protocol was approved by the Bioethics Committee at the Jagiellonian University in Cracow, Poland.
The patients were divided into two groups: patients with active CD (n = 25) and patients with inactive disease (remission; n = 22). The diagnosis of CD was based on clinical, radiological, endoscopic and histopathological criteria (1). Deterioration of CD was classified according to the CD activity index (CDAI) (34), which is a composite scoring system based on selected clinical symptoms, such as the number of liquid stools, the severity of abdominal pain, general well-being, extraintestinal CD manifestations, abdominal mass, use of antidiarrheal drugs, as well as haematocrit and body weight. CDAI values of 150 and below are associated with remission, and values above indicate active disease (150 – 219 points - a mild exacerbation, moderate 220 – 450, above 450 points as severe) (35). Patients were placed on maintenance therapy with azathioprine (2 – 2.5mg/kg/day) according to the ECCO guidelines (2). All patients with active and inactive CD and localization of inflammatory lesions in the large intestine and the ileocecal region were treated with mesalamine (2 g/day). The control group comprised 25 healthy volunteers (mean age 34.2 ± 9.8 years; 13 males and 12 females).
Exclusion criteria were the following: any known systemic infection or disease, pregnancy or lactation, alcoholism, tobacco smoking, use of antibiotics, antioxidants (e.g., vitamins C, E) or anti-inflammatory drugs within the last 6 months, periodontal disease, presence of an oral mucosal inflammatory condition (e.g. aphthae, lichen planus, leucoplakia), removable orthodontic appliances, and symptoms of acute illness (e.g., fever, sore throat).
Methods
Patient demographics, clinical features, results of GI tract examinations, localisation of CD inflammatory changes in the GI tract, CD history, and treatment were recorded. Samples of blood and stimulated whole saliva were collected from fasted participants in the morning hours between 8:00 AM and 10:00 AM for the further analyses.
Material preparation and storage
Blood sampling
Blood samples were collected from the ulnar vein in a closed Sarstedt systems (Sarstedt AG & Co., Numbrecht, Germany) that contained as required, either EDTA as an anticoagulant, or no anticoagulant. Serum and plasma were carefully separated by centrifugation at 3000 rpm for 10 min. at 4°C. The plasma samples were stored at -80°C until SOD and GPx were assayed. Routine laboratory tests included: complete blood counts, platelets, C-reactive protein (CRP) in serum, and these were measured in the hospital laboratory using standard procedures.
Saliva samples
Saliva samples were collected from all participants using a Salivette® Cotton Swab system (Sarstedt AG & Co., Numbrecht, Germany). The subject rinsed their mouth with tap water for 30 s and expectorated it before saliva collection, placed the cotton swab in the mouth and chew it for 60 s to stimulate salivation. Salivette with swab saturated with saliva were centrifuged at 1000 rpm for 2 min at 4°C. Clear saliva samples (about 1 ml) were immediately aliquoted to sterile 0.2 ml micro test tubes, Eppendorf type, and frozen at -80°C until assayed.
Assays for glutathione peroxidase and superoxide dismutase
GPx activity in plasma and saliva were measured by the colorimetric assay using a Glutathione Peroxidase Assay Kit (Cayman Chemical Company, Ann Arbor, USA) according to the manufacturer's instruction. The assay kit measures GPx activity indirectly by a coupled reaction with glutathione reductase. Oxidized glutathione is produced upon reduction of hydroperoxide by GPx and is recycled to its reduced state by glutathione reductase and nicotinamide adenine dinucleotide phosphate (NADPH).
The principle behind the assay is that oxidation of NADPH to NADP+ is accompanied by a decrease in absorbance at 340 nm. Under conditions in which the GPx activity is rate limiting, the rate of decrease in the A340 is directly proportional to the GPx activity in the sample (35). The absorbance was monitored once every minute at 340 nm using a plate reader to obtain 5 time points. One unit of GPx was defined as the amount of enzyme causing oxidation od 1 nM of NADPH to NADP+ per min. at 25°C (36).
SOD activity in plasma and saliva was measured by the colorimetric assay using a Superoxide Dismutase Assay Kit (Cayman Chemical Company, Ann Arbor, USA) according to the manufacturer's instruction. The SOD assay measures three types of SOD (copper/zinc, manganese, and iron). SOD activity was assayed by measuring the dismutation of superoxide radicals generated by xanthine oxidase and hypoxanthine. The absorbance was monitored at 440 – 460 nm using a plate reader.
One unit of SOD was defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical. The activity of GPx and SOD were measured in duplicate in the Laboratory of Biochemistry of the 2nd Department of Internal Medicine, Jagiellonian University in Cracow.
Statistical analysis
A statistical analysis was performed using Statistica 10.0 software (StatSoft, Inc., Tulsa, Oklahoma, United States). The Shapiro-Wilk test was used to test the normality of data distribution. Those results with a Gaussian distribution were analysed with the Student's t-test. The nonparametric Mann-Whitney U test and the Kruskal-Wallis test were used to compare different group variables. The results were presented as percentage for categorical variables, as mean with standard deviation (S.D.) for normally distributed variables, or as median with interquartile range for not normally distributed continuous variables. The relationships between clinical data, CD activity, plasma and salivary parameters were evaluated using the Spearman's rank correlation coefficient. All statistical tests were considered significant at the 0.05 probability level.
RESULTS
Patients' characteristics
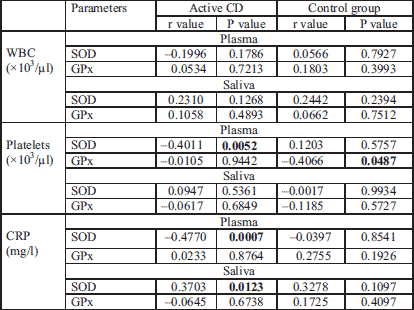
The demographic, clinical and laboratory data are summarized in Table 1. As expected, patients with active CD had a significantly lower red blood cell count and haemoglobin concentration in blood, higher platelets count and higher CRP level in serum compared with patients with inactive CD and controls. The mean CDAI of patients with active CD was 262.5 points, and for those with inactive disease this was 67.2 points (P < 0.001) (Table 1). Most patients (73%) had moderate disease activity, the remaining 27% had low active CD. The localisation of intestinal lesions is presented in Table 1. None of patients had previously been operated on because of CD.
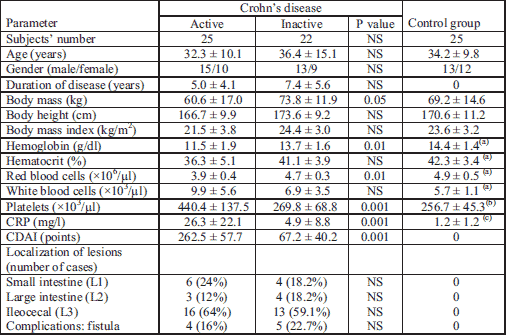
Superoxide dismutase and glutathione peroxidase activities in plasma and saliva
The results of the plasma and saliva analysis of patients with CD and the control groups are presented in Table 2. The analysis of the plasma in this cohort revealed a significant decrease in the SOD activity in active CD as compared to inactive CD (P = 0.0029) and the control group (P = 0.034). The results showed no significant differences in the salivary activities of SOD in active CD versus inactive CD and the control group. The results of GPx activities in plasma and saliva in active and inactive CD as well as controls were not statistically significant. The activities of SOD, and particularly GPx, were significantly lower in saliva compared to serum values (P < 0.0005).
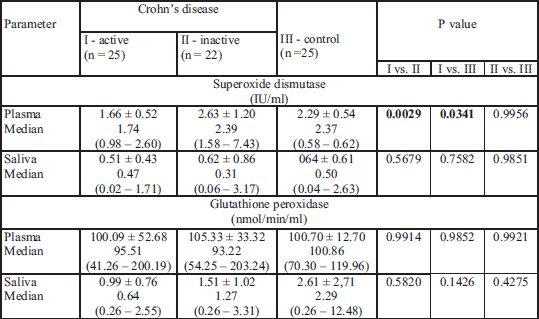
Correlations between SOD, GPx and selected clinical features are presented in Table 3. A positive correlation was observed between the duration of CD and elevated GPx activity in plasma. No associations were observed in respect to SOD activities measured in plasma and saliva of patients with CD.
The correlation was found between body mass index (BMI) values and elevated SOD activity in plasma of patients with CD. Neither SOD activity in saliva nor GPx activity in both plasma and saliva correlated with BMI.
CDAI scores correlated inversely with SOD in plasma but not in saliva. No associations we observed in respect to GPx activities in both plasma and saliva and CDAI. Higher activity of plasma SOD was observed in patients with inactive CD in comparison with active CD according to CDAI (P = 0.004).
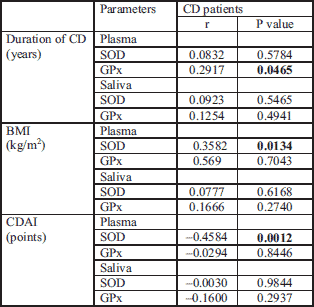
The number of blood platelets in CD patients correlated with the SOD activity in plasma (r = – 0.4; P = 0.005). GPx activity in plasma and saliva did not correlated with platelets in patients with CD, however, a positive correlation was noted in plasma of the control group.
CRP level in the CD group correlated inversely with SOD activity in plasma (r = – 0.5; P = 0.0007) and with SOD in saliva (r = 0.37; P = 0.0123). No associations were observed between CRP and GPx activity in plasma and saliva.
Statistically significant higher activity of plasma SOD was observed in CD patients with fistulas in comparison with patients without fistulas (1.86 ± 0.88 vs. 1.66 ± 0.52 U/ml, respectively; P = 0.03). According to the presence of fistulas in CD patients, there were no statistically significant differences in plasma or saliva GPx activity. No association was also found between SOD and GPx activities in plasma or saliva and the intestinal localization of CD.
DISCUSSION
This study demonstrated the lower activity of SOD in plasma of patients with the active form of CD in comparison to patients in remission from the disease. The results suggest that in active CD the antioxidant defence system is diminished and return to normal value in remission. Assessment of SOD activity may have both diagnostic and prognostic value, as well.
The main antioxidant enzymes are SOD, GPx and catalase, however in human plasma there is very little or no catalase activity (6, 7). Antioxidant defences both within cells and extracellularly should inhibit the toxic effects of lipid peroxides and maintain normal physiology. The major role of plasma antioxidant defence is to bind metal ions and therefore to lower their plasma levels and capacity to stimulate ROS, and to prevent the formation of hydroxyl radicals, which can generate lipid peroxides (7). OxS could be a major factor to the tissue damage and fibrosis that are characteristic for CD (9, 14). An imbalance between increased ROS levels and decreased antioxidant defences was described in CD patients by many authors (7, 11, 12, 22). Decreased blood and mucosal levels of antioxidants such as vitamins C, E and β-carotene have been reported in CD patients (7).
In the active CD SOD activity in plasma was decreased compared to the inactive CD, but the results of GPx activities in plasma in both CD groups as well as controls did not reveal any statistically significant differences. Changes in SOD activity were not observed in the whole stimulated saliva of CD patients. We also observed significantly lower SOD, and GPx activity in the whole stimulated saliva.
The results of SOD activity measured in plasma of patients with CD have been demonstrated to be diverse (7, 14). In a recent study, Alzoghaibi et al. have shown significantly lower plasma antioxidant activity for SOD in CD patients than is the case controls, and they concluded that these patients are more susceptible to OxS (11). We compared the activity of SOD in plasma of patients with active CD with various clinical factors. The Spearman correlation presented the positive relationship between SOD and BMI, negative correlation with CDAI scores, and the number of blood platelets as well as with CRP levels in serum.
The present study confirmed the significantly lower activities of SOD in plasma of patients with active CD. This results are highly dependent on the clinical activity of the disease and the therapy. This might be the source of the discrepancies in study results observed by some authors (14). Incorrectly treated or untreated CD leads to an intensification of the OxS, leading to exhaustion of the enzymatic activities of the elements of antioxidant defence as a result of radical damage to proteins, nucleic acids and free fatty acids. Also, an enzyme located in the mucous membrane of the intestines, or enterocytes, which are more susceptible to oxidant damage, can be inactivated to a larger extent leading to variation in the results of its determination. Intestinal complications of CD, such as fistula, abscesses or stenosis formation, might be also related to the decrease in the enzymatic activity of organ tissue SOD (7).
In our studies SOD activity was decreased only in plasma of active CD patients, what stays in contrast with findings of Kolacek et al. who observed decreased SOD activity in paediatric patients with CD remission (37). However, this is consistent with the observation that patients in the active CD have elevated OxS and reduced antioxidant defence parameters, while for the those in the remission CD these parameters are comparable with those of healthy controls (38, 39).
Except for the activity of SOD and GPx levels in plasma, we have not found any significant differences in these parameters of saliva between patients and the control group. Although saliva is a promising material for testing many biochemical substances, it is not specific for the measurement of intracellular enzyme activities.
Results of GPx and SOD activities measured in CD patients have been demonstrated to be diverse when analysing plasma samples (14). Plasma GPx activity was higher in CD patients compared to controls, but the differences were not seen in patients with inactive CD, without signs of disease symptoms (21). In another study, no differences of plasma GPx activity were observed between patients with active or inactive CD and controls (40, 41). The different results in various publications indicate that it is important to consider many factors affecting the results of OxS markers, including the duration of CD, active or inactive disease, specific medications, the type of the sample, and the time point when it is collected (14).
The current study has shown that SOD may have diagnostic and prognostic value. Changes in SOD activity in plasma confirmed former reports that the antioxidant system is impaired in active CD (12). SOD activity measurement might have the clinical potential, and could be used as another prognostic marker related to the OxS reactions in CD patients. Further research with a larger sample would increase the statistical significance of the tests and the value of the research. The potential positive results from such a study are strongly suggested by our results.
Finally, saliva as an easy obtainable body fluid, has the potential diagnostic properties. It could be used to help diagnose not only oral diseases, but also be applied in the diagnosis of systemic conditions (42-44). Saliva might be employed as a non-invasive diagnostic fluid to measure biomarkers released during the course of a disease (45-47), including indicators of oxidative processes (48). Therefore, characterization of the specific salivary biomarkers associated with presence of CD and its severity could have a major impact on the diagnosis and monitoring of this disease (49). Jahanshahi et al. analysed the whole saliva of patients with CD and found that the saliva was oxidatively stressed with increased values of nitric oxide and lipid peroxide markers as well as decreased antioxidant power assessed by ferric reducing ability (50). Rezaie et al. measured oxidative capacity, and several specific antioxidants in saliva, e.g. uric acid, albumin, transferrin, thiol molecules as well as lipid peroxidation, nitric oxide and TGF-beta in patients with active CD (13). They observed significant reduction in the salivary levels of total antioxidant capacity, albumin and uric acid (13). Moreover, in their study CDAI correlated with antioxidant capacity and lipid peroxidation (13).
In this study, we performed a parallel assessment of SOD and GPx activities in plasma as well as in the whole stimulated saliva in patients with various activities of CD compared to the control group, but we were not able to obtain any conclusive results. The activities of both enzymes were much lower in saliva in comparison to plasma levels, and on basis of these observations we would not recommend the use of saliva to assay SOD or GPx instead of plasma in patients with CD. However, a study on a larger group of patients might change our approach; as well it is possible that the results might be different in a group of patients presenting oral cavity changes, which are not rarely seen in CD patients. It should be mention that patients with changes in the oral cavity were not included in this research.
There are some limitations to the study that should be mentioned. First, other parameters of the inflammation process, OxS, antioxidant defence factors were not studied. Second, all patients with CD were chronically treated with azathioprine, and patients with lesions located in the large intestine and in the ileocecal region were treated with mesalamine (5-ASA). Therefore, we could not exclude the effect of the medication on the SOD and GPx activities measured in plasma and saliva. 5-ASA is a potent antioxidant drug, promotes mucosal healing, it is used in active CD and as the maintenance treatment of the disease with a large intestine localization, and reduces the risk of colon cancer which prevalence is increased in IBD patient. Third, a study with larger groups of patients would increase the statistical significance of the tests and the value of the research.
In conclusion, in active CD with higher intensification of OxS, the antioxidant defence system is diminished and returns to normal values in remission. Results of SOD and GPx assayed in the saliva of patients with CD are not conclusive, suggesting that saliva, however easy to obtain, seems to be not an appropriate material for further similar studies.
List of abbreviations: BMI, body mass index; CD, Crohn's disease; CDAI, Crohn's disease activity index; CRP, C-reactive protein; GI, gastrointestinal; GPx, glutathione peroxidase; NADPH, nicotinamide adenine dinucleotide phosphate; OxS, oxidative stress; ROS, reactive oxygen species; SOD, superoxide dismutase
Acknowledgements: This study was supported by grants from the Jagiellonian University Medical College in Cracow, Poland.
Conflict of interests: None declared.
REFERENCES
- Van Assche G, Dignass A, Panes J, et al. European Crohn's and Colitis Organisation (ECCO) The Second European Evidence-Based Concensus on the Diagnosis and Management of Crohn's: Definitions and diagnosis. J Crohns Colitis 2010; 4: 7-27.
- Dignass A, Van Assche G, Lindsay JO, et al. European Crohn's and Colitis Organisation (ECCO). The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis 2010; 4: 28-62.
- Burisch J, Munkholm P. The epidemiology of inflammatory bowel disease. Scand J Gastroenterol 2015; 50: 942-951.
- Wallace KL, Zheng L-B, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol 2014; 20: 6-21.
- Moret I, Cerrillo E, Navarro-Puche A, et al. Oxidative stress in Crohn's disease. Gastroenterol Hepatol 2014; 37: 28-34.
- Piechota-Polanczyk A, Fichna J. The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch Pharmacol 2014; 387: 605-620.
- Alzoghaibi MA. Concepts of oxidative stress and antioxidant defense in Crohn's disease. World J Gastroenterol 2013; 19: 6540-6547.
- Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 2014; 94: 329-354.
- Hagel AE, de Rossi T, Konturek PC, et al. Plasma histamine and tumour necrosis factor-alpha levels in Crohn's disease and ulcerative colitis at various stages of disease. J Physiol Pharmacol 2015; 66: 549-556.
- Pereira C, Gracio D, Teixeira JP, Magro F. Oxidative stress and DNA damage: implications in inflammatory bowel disease. Inflamm Bowel Dis 2015; 21: 2403-2417.
- Alzoghaibi MA, Al-Mofleh IA, Al-Jebreen AM. Antioxidant activities for superoxide dismutase in patients with Crohn's disease. J Basic Clin Physiol Pharm 2014; 25: 59-62.
- D'Odorico A, Bortolan S, Cardin R, et al. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand J Gastroenterol 2001; 36: 1289-1294.
- Rezaie A, Parker RD, Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause. Dig Dis Sci 2007; 52: 2015-2021.
- Moret-Tatay I, Iborra M, Cerrillo E, Tortosa L, Nos P, Beltran B. Possible biomarkers in blood for Crohn's disease: oxidative stress and microRNAs - current evidences and further aspects to unravel. Oxid Med Cell Longev 2016; 2016: 2325162. doi: 10.1155/2016/2325162.
- Owczarek D, Cibor D. Mach T. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), arginine, and 8-iso-prostaglandin F2alpha (8-iso-PGF2alpha) level in patients with inflammatory bowel diseases. Inflamm Bowel Dis 2010; 16: 52-57.
- Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol 2003; 201: 28-36.
- Moura FA, de Andrade KQ, dos Santos JCF, Araujo OR, Goulart MO. Antioxidant therapy for treatment of inflammatory bowel disease: does it work? Redox Biol 2015; 6: 617-639.
- Abiodun OO, Rodriguez-Nogales A, Algieri F, et al. Antiinflammatory and immunomodulatory activity of an ethanolic extract from the stem bark of Terminalia catappa L. (Combretaceae): in vitro and in vivo evidences. J Ethnopharmacol 2016; 192: 309-319.
- Palla AH, Iqbal NT, Minhas K, Gilani AH. Flaxseed extract exhibits mucosal protective effect in acetic acid induced colitis in mice by modulating cytokines, antioxidant and antiinflammatory mechanisms. Int Immunopharmacol 2016; 38: 153-166.
- Khan RA, Mallick N, Feroz Z. Anti-inflammatory effects of Citrus sinensis L., Citrus paradisi L. and their combinations. Pak J Pharm Sci 2016; 29: 843-852.
- Maor I, Rainis T, Lanir A, Lavy A. Oxidative stress, inflammation and neutrophil superodixe release in patients with Crohn's disease: distinction betweensactive and non-active disease. Dig Dis Sci 2008; 53: 2208-2214.
- Lih-Brody L, Powell SR, Collier KP, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci 1996; 41: 2078-2086.
- Pinto MA, Lopes MS, Bastos ST, et al. Does active Crohn's disease have decreased intestinal antioxidant capacity? J Crohns Colitis 2013; 7: e358-e366. doi: 10.1016/ j.crohns.2013.02.010.
- Rezaie A, Ghorbani F, Eshghtork A, et al. Alterations in salivary antioxidants, nitric oxide, and transforming growth factor-beta 1 in relation to disease activity in Crohn's disease patients. Ann NY Acad Sci 2006; 1091: 110-122.
- Ruseva B, Atanasova M, Tsvetkova R, et al. Effect of selenium supplementation on redox status of the aortic wall in young spontaneously hypertensive rats. Oxid Med Cell Longev 2015; 2015: 609053. doi: 10.1155/2015/609053
- Stanley JA, Neelamohan R, Suthagar E, et al. Lipid peroxidation and antioxidants status in human malignant and non-malignant thyroid tumours. Hum Exp Toxicol 2016; 35: 585-597.
- Giustarini D, Tsikas D, Colombo G, et al. Pitfalls in the analysis of the physiological antioxidant glutathione (GSH) and its disulfide (GSSG) in biological samples: an elephant in the room. J Chromatogr B Analyt Technol Biomed Life Sci 2016; 1019: 21-28.
- Singh A, Jahan N, Radhakrishnan G, Banerjee BD. To evaluate the efficacy of combination antioxidant therapy on oxidative stress parameters in seminal plasma in the male infertility. J Clin Diagn Res 2016; 10: QC14-QC17. doi: 10.7860/JCDR/2016/15597.8159
- Stachniuk J, Kubalczyk P, Furmaniak P, Glowacki R. A versatile method for analysis of saliva, plasma and urine for total thiols using HPLC with UV detection. Talanta 2016; 155: 70-77.
- Moniuszko-Malinowska A, Luczaj W, Jarocka-Karpowicz I, et al. Lipid peroxidation in the pathogenesis of neuroborreliosis. Free Radic Biol Med 2016; 96: 255-263.
- Mussavira S, Dharmalingam M, Omana Sukumaran B. Salivary glucose and antioxidant defense markers in type II diabetes mellitus. Turk J Med Sci 2015; 45: 141-147.
- Said HS, Suda W, Nakagome S, et al. Dysbiosis of salivary microbiota in inflammatory bowel disease and its association with oral immunological biomarkers. DNA Res 2014; 21: 15-25.
- Ben Ali MJ, Guesmi F, Harrath AH, et al. Investigation of antiulcer and antioxidant activity of Juniperus phoenicea L. (1753) essential oil in an experimental rat model. Biol Pharm Bull 2015; 38: 1738-1746.
- Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 1976; 70: 439-444.
- Ismail NA, Okasha SH, Dhawan A, Abdel Rahman AO, Hamid NA, Shaker O. Glutathione peroxidase, superoxide dismutase and catalase activities in children with chronic hepatitis. Adv Biosc Biotech 2012; 3: 972-977.
- Gumus P, Emingil G, Ozturk VO, Belibasakis GN, Bostanci N. Oxidative stress markers in saliva and periodontal disease status: modulation during pregnancy and postpartum. BMC Infect Dis 2015; 15: 261.
- Kolacek M, Muchova J, Dvorakova M, et al. Effect of natural polyphenols (Pycnogenol) on oxidative stress markers in children suffering from Crohn's disease- a pilot study. Free Radic Res 2013; 47: 624-634.
- Wendland BE, Aghdassi E, Tam C, et al. Lipid peroxidation and plasma antioxidant micronutrients in Crohn disease. Am J Clin Nutr 2001; 74: 259-264.
- Koutroubakis IE, Malliaraki N, Dimoulios PD, Karmiris K, Castanas E, Kouroumalis EA. Decreased total and corrected antioxidant capacity in patients with inflammatory bowel disease. Dig Dis Sci 2004; 49: 1433-1437.
- Jain SK. Superoxide dismutase overexpression and cellular oxidative damage in diabetes. A commentary on "Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress". Free Radic Biol Med 2006; 41: 1187-1190.
- Akman T, Akarsu M, Akpinar H, Resmi H, Sezar E. Erythrocyte deformality and oxidative stress in inflammatory bowel disease. Dig Dis Sci 2012; 57: 458-464.
- Teles RP, Likhari V, Socransky SS, Haffajee AD. Salivary cytokine levels in chronic periodontitis and periodontally healthy subjects. A cross-sectional Study. J Periodontal Res 2009; 44: 411-417.
- Wong DT. Salivary diagnostics powered by nanotechnologies, proteomics and genomics. J Am Dent Assoc 2006; 137: 313-321.
- Hu S, Loo JA, Wong DT. Human saliva proteome analysis and disease biomarker discovery. Expert Rev Proteomics 2007; 4: 531-538.
- Kaufman E, Lamster IB. Analysis of saliva for periodontal diagnosis - a review. J Clin Periodontol 2000; 27: 453-465.
- Lamster IB, Grbic JT. Diagnosis of periodontal disease based on analysis of the host response. Periodontol 2000 1995; 7: 83-99.
- Taba M, Kinney J, Kim AS, Giannobile WV. Diagnostic biomarkers for oral and periodontal diseases. Dent Clin North Am 2005; 49: 551-571.
- Buczko P, Zalewska A, Szarmach I. Saliva and oxidative stress in oral cavity and in some systemic disorders. J Physiol Pharmacol 2015; 66: 3-9.
- Szczeklik K, Owczarek D, Pytko-Polonczyk J, Kesek B, Mach TH. Proinflammatory cytokines in the saliva of patients with active and non-active Crohn's disease. Pol Arch Med Wewn 2012; 122: 200-208.
- Jahanshahi G, Motavasel V, Rezaie A, Hashtroudi AA, Daryani NE, Abdollahi M. Alterations in antioxidant power and levels of epidermal growth factor and nitric oxide in saliva of patients with inflammatory bowel diseases. Dig Dis Sci 2004; 49: 1752-1757.
A c c e p t e d : October 31, 2016